Research Briefs
Read about NIH scientific advances and discoveries by intramural scientists:
- NIEHS: MALE HORMONES MODULATE GASTRIC INFLAMMATION IN MICE
- NCATS: SMALL-MOLECULE COCKTAIL PROTECTS STEM CELLS FROM STRESS
- NIAAA: NEURAL CIRCUIT COMPETES TO INFLUENCE THE PERSISTENCE OF TRAUMATIC MEMORIES
- NIAID: SALMONELLA EXPLOITS INTESTINAL CELL DEFENSE TO AID GUT COLONIZATION
- NIDDK, NCI: KEY PROTEIN ENHANCES BETA-CELL ACTIVITY, IMPROVES GLUCOSE HOMEOSTASIS IN TYPE 2 DIABETES
- NINDS, NICHD: GENETIC FORM OF CHILDHOOD ALS DISCOVERED
- NIDDK, NHLBI: CLUES TO THE EFFICACY OF HBV TREATMENT WITH PEGYLATED INTERFERON-ALPHA
NIEHS: MALE HORMONES MODULATE GASTRIC INFLAMMATION IN MICE
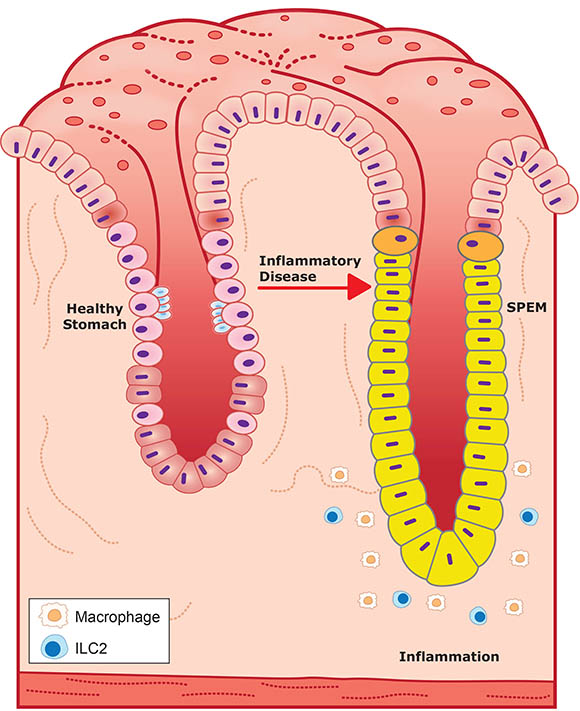
CREDIT: NIEHS
Glucocorticoids and androgens promote a healthy stomach pit (stomach pits are glands embedded in the lining of the stomach) by inhibiting inflammation, left, while their absence promotes inflammation and SPEM seen in a diseased pit, right. SPEM glands are also much larger than healthy stomach glands.
In a recent study published in Gastroenterology, scientists at NIEHS reported that androgens, or male sex hormones, play a critical role in regulating gastric inflammation. Chronic gastric inflammation is known to lead to a precancerous condition in the stomach called spasmolytic polypeptide-expressing metaplasia (SPEM). Women suffer more frequently than men from inflammatory diseases, and this study sheds new light on the action of androgens as an inflammatory mediator.
Researchers first removed the adrenal glands, which secrete inflammation-suppressing hormones such as glucocorticoids, from both male and female mice. The removal resulted in spontaneous gastric inflammation and SPEM in females but not in males. However, removal of both glucocorticoids and androgens abolished the male protective effects, triggering stomach inflammation equally in all mice. Additionally, treatment of female mice with androgens prevented gastric inflammation and SPEM and even reversed the pathology after the onset of the disease.
“The study showed that glucocorticoids and androgens act like brake pedals on the immune system and are essential for regulating stomach inflammation,” said lead author Jonathan Busada. The investigators offered a possible explanation for this effect, noting that special kinds of immune cells called type 2 innate lymphoid cells (ILC2s) play a key role in detecting hormones in the stomach. When both hormones are absent in a diseased stomach, ILC2s signal other immune cells that promote further damage to gastric glands, causing inflammation and, ultimately, cancer. “ILC2s are the only immune cells that contain androgen receptors and could be a potential therapeutic target,” said co-corresponding author John Cidlowski. (NIH authors: J.T. Busada, K.N. Peterson, X. Xu, R.H. Oakley, D.N. Cook, and J.A. Cidlowski, Gastroenterology 2021; DOI:10.1053/j.gastro.2021.04.075)
NCATS: SMALL-MOLECULE COCKTAIL PROTECTS STEM CELLS FROM STRESS
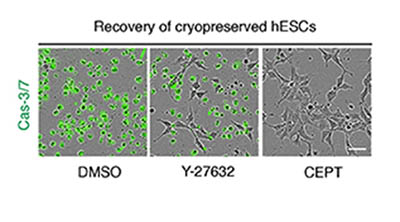
CREDIT: CHEN ET AL., NATURE METHODS
NCATS scientists have devised a small-molecule cocktail called CEPT that helps protect human pluripotent stem cells from potential DNA damage due to the stresses of being grown in a dish. Here, frozen human stem cells were thawed, placed on cell culture plates in the presence of different reagents, and analyzed 12 hours later. In the left and center panels, Caspase 3/7 (green), a marker for dead cells, indicates poor survival of stem cells treated with a control (DMSO) and Y-27632, a standard compound. In contrast, the right panel shows that virtually all cells survived in the presence of the CEPT cocktail.
Researchers at NCATS found that a combination of four drugs and compounds can protect induced pluripotent stem cells (iPSCs) from stresses commonly encountered when the cells are in a laboratory setting. These stem cells can theoretically grow forever: They can be used in genome editing and have the potential to treat a myriad of disorders including Parkinson disease, Alzheimer disease, and spinal-cord injury. However, stem cells are sensitive and can be easily damaged during the research process, posing a challenge to scientists.
A team of investigators led by Ilyas Singeç created a small-molecule cocktail, which they named CEPT, that greatly improves iPSC survival. The researchers first identified a molecule, Chroman 1, that strongly inhibited an enzyme involved in iPSC stress and worked synergistically with the investigational drug emricasan. Follow-up experiments were then used to model the conditions of single-cell cloning, a particularly stressful—and critical—use for iPSCs. In this step, a third compound called trans-ISRIB was identified, which was enhanced with a commercially available polyamine mixture that further optimized cell viability.
The new cocktail proved useful for several common iPSC research tasks. For example, cells can be damaged when they are frozen for transport and later thawed for clinical use. Treatment with CEPT resulted in significantly better cell-survival outcomes than existing protocols during this cryopreservation process. The authors noted potential applications for this discovery across many biomedical fields including regenerative medicine and drug development. “Our approach could improve safety and ensure that the next-generation stem-cell lines are cultured at high quality before moving into the clinic,” said Singeç. (NIH authors: Y. Chen, C.A. Tristan, L. Chen, V.M. Jovanovic, C. Malley, P. Chu, S. Ryu, T. Deng, P. Ormanoglu, D. Tao, Y. Fang, J. Slamecka, H. Hong, C.A. LeClair, S. Michael, C.P. Austin, A. Simeonov, and I. Singeç, Nat Methods 18:528–541, 2021; DOI:10.1038/s41592-021-01126-2)
NIAAA: NEURAL CIRCUIT COMPETES TO INFLUENCE THE PERSISTENCE OF TRAUMATIC MEMORIES
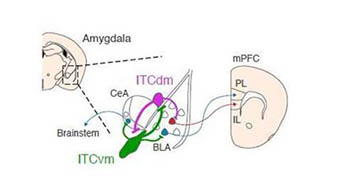
CREDIT: KENTA M. HAGIHARA, FRIEDRICH MIESCHER INSTITUTE FOR BIOMEDICAL RESEARCH (BASEL, SWITZERLAND)
Distinct clusters of neurons located in the amygdala modulate high and low fear states via connections to other regions in the brain.
NIH scientists and their colleagues in the United States, Switzerland, and Germany have characterized key brain areas involved in the formation and loss of traumatic memories. When a person experiences trauma, their brain undergoes a process called persistence in which negative feelings become linked to cues (specific elements of the trauma) and continue to provoke fearful behavior. Over time, exposure to trauma-associated cues without any adverse consequence leads to extinction in which the harmful association is forgotten.
The amygdala, known as the emotional processing region of the brain, is surrounded by distinct clusters of neurons called intercalated cells (ITCs). The researchers used in vivo calcium imaging to measure the activity of these clusters in mice during the simultaneous presentation of a mildly painful shock and a harmless auditory cue. The investigators then removed the painful stimuli and observed brain activity when the auditory cue was presented alone, such as occurs during extinction. The ITCs were found to directly compete with each other for influence in a process called mutual synaptic inhibition, in which each distinct cluster could dial up—or down—the fear state. Furthermore, the study showed that this neural circuit linked the amygdala to other fear-regulating regions in the midbrain and prefrontal cortex.
According to lead researcher Andrew Holmes, studies targeting these brain areas in humans could give doctors tools to identify patients at higher risk for conditions such as post-traumatic stress disorder and could provide insight into treatments for the persistence of traumatic memories. “This finding now raises interesting questions about whether dysfunction of this brain system could contribute to the marked individual differences in risk for trauma-related psychiatric disorders,” said Holmes. (NIH authors: O. Bukalo, A. Limoges, T. Rigg, T. Campbell, A. Mendez, C. Weinholtz, and A. Holmes, Nature 594:403–407, 2021; DOI:10.1038/s41586-021-03593-1)
NIAID: SALMONELLA EXPLOITS INTESTINAL CELL DEFENSE TO AID GUT COLONIZATION
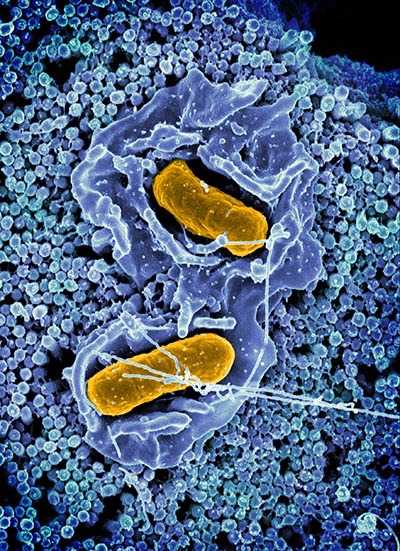
CREDIT: NIAID
Scanning electron micrograph of Salmonella Typhimurium invading a human epithelial cell.
NIAID scientists have found that the immune response aimed at removing Salmonella bacteria from the gastrointestinal tract could instead be helping the bacteria to colonize the gut, where it can persist and be shed via feces into the environment and transmitted to new hosts.
A common cause of gastroenteritis in humans, Salmonella bacteria colonize the gut by invading cells lining the intestines, called intestinal epithelial cells (IECs), and forming a contained space (vacuole). The bacteria can either remain within the vacuole or escape into the cell fluid (cytosol). Cytosolic bacteria trigger the IECs to eject themselves into the gut lumen, expelling the infected cell from the host in a protective response to limit replication and tissue penetration. However, previous studies have shown cytosolic Salmonella to not only rapidly replicate, but also feature adaptations that aide the bacteria’s invasion into new cells. This discovery led the scientists to question whether the bacteria were exploiting the host’s defense strategy to amplify its own ability to chronically infect.
To test this hypothesis, the investigators genetically modified Salmonella bacteria to self-destruct when exposed to the IEC cytosol, but grow normally elsewhere. Indeed, the mice infected with the self-destructing version showed reduced bacterial colonization and fecal shedding. According to the investigators, this finding is an example of bacteria evolving to adapt to pressure exerted by the host immune system. They propose that Salmonella bacteria use these rounds of invasion, replication, and release from ejected IECs to allow them to colonize the gut and seed the intestine for chronic fecal shedding of the bacteria. These insights could offer new directions to treat this common pathogen. (NIH authors: A. Chong, K.G. Cooper, L. Kari, O.R. Nilsson, C. Hillman, B.A. Fleming, Q. Wang, V. Nair, and O. Steele-Mortimer, Cell Host Microbe 2021; DOI:10.1016/j.chom.2021.04.017)
NIDDK, NCI: KEY PROTEIN ENHANCES BETA-CELL ACTIVITY, IMPROVES GLUCOSE HOMEOSTASIS IN TYPE 2 DIABETES
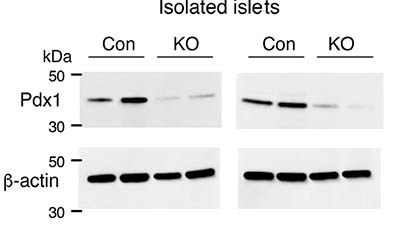
CREDIT: BARELLA ET AL., NATURE COMMUNICATIONS
Reduced expression of Pdx1 protein in the absence of beta-cell barr1. Lysates from pancreatic islets derived from HFD control (Con) and beta-barr1-KO (KO) mice were subjected to Western blotting studies. Blots were probed with anti-Pdx1 and anti-β-actin antibodies.
Type 2 diabetes (T2D) is a disease characterized by high blood-glucose concentrations, which are caused by two major factors: pancreatic beta cells do not produce enough insulin; and peripheral tissues such as fat, liver, or skeletal muscle do not properly respond to circulating insulin (insulin resistance). Insulin resistance is known to occur in individuals with obesity and usually triggers an increase in beta-cell mass that can delay or prevent the development of T2D.
In this study, NIH scientists identified the beta-arrestin-1 protein (Barr1) as an essential factor in the proper functioning of pancreatic beta cells. The team genetically modified mice to inactivate the gene that codes for Barr1 specifically in beta cells. These knockout mice were fed a high-fat diet alongside normal mice to induce obesity. Compared with their control littermates, the knockout mice exhibited a significant increase in blood-glucose concentration and reduction in plasma insulin in both fed and fasting conditions. Additional studies indicated that Barr1 plays a critical role in mediating the compensatory increase in beta-cell mass that occurs in the obese state.
Moreover, the authors found that Barr1 appears to promote the expression of a protein called pancreatic and duodenal homeobox 1, a transcription factor that plays a key role in pancreatic development and the expansion of beta-cell mass—a finding the authors also confirmed in human beta-cell lines. “The results of this study support strategies to enhance Barr1 activity in beta cells as a therapeutic option for restoring glucose homeostasis and preventing T2D,” said co-author Jürgen Wess. (NIH authors: L.F. Barella, M. Rossi, S.P. Pydi, J. Meister, S. Jain, Y. Cui, O. Gavrilova, G. Fulgenzi, L. Tessarollo, and J. Wess, Nat Commun 12:Article number 3385, 2021; DOI:10.1038/s41467-021-23656-1)
NINDS, NICHD: GENETIC FORM OF CHILDHOOD ALS DISCOVERED
CREDIT: NINDS
NIH researchers discovered a new form of ALS that begins in childhood. The study linked the disease to a gene called SPLTC1. As part of the study, NIH senior scientist Carsten Bönnemann (right) examined a young patient from the Apulia region of Italy.
A team of international researchers led by NIH scientists and the Uniformed Services University (Bethesda, Maryland) have identified the gene responsible for a rare form of amyotrophic lateral sclerosis (ALS) in children—and have developed a potential treatment strategy.
The authors studied 11 children from around the globe with undiagnosed ALS-like symptoms, such as severely weakened or paralyzed muscles. Compared with sporadic adult ALS, the children’s symptoms progressed more slowly but began as early as four years of age. The researchers analyzed the patients’ DNA using whole-exome sequencing and found that they each had a mutation (either inherited or spontaneous) in SPLTC1, a gene associated with producing a class of fats called sphingolipids. Previously reported mutations in SPLTC1 typically prompt the enzyme serine palmitoyltransferase (SPT) to overproduce toxic versions of these fats, causing a disease called hereditary sensory and autonomic neuropathy type 1.
In the children, however, the mutation appeared to result in elevated plasma concentrations of normal sphingolipids caused by unchecked SPT activity. The investigators then were able to show that the SPLTC1 variant prevented the protein ORMDL from inhibiting SPT, disrupting the typical feedback loop that dictates sphingolipid homeostasis.
The scientists recognized a potential for treatment and created small interfering strands of RNA to target the SPLTC1 mutation in vitro. Using the patient’s skin cells, they tested the interfering RNAs and found they were effective in decreasing expression of the problematic gene and that sphingolipid synthesis returned to normal rates.
“These preliminary results suggest that we may be able to use a precision gene-silencing strategy to treat patients with this type of ALS,” said the study’s senior author, Carsten Bönnemann. “Our ultimate goal is to translate these ideas into effective treatments for our patients who currently have no therapeutic options.” (NIH authors: P. Mohassel, S. Donkervoort, M. Nalls, A.R. Foley, Y. Hu, C. Grunseich, A.R. Nickolls, N. Pourshafie, S.B. Neuhaus, D. Saade, Z. Piccus, C.E. LePichon, and C.G. Bönnemann, Nat Med 2021; DOI:10.1038/s41591-021-01346-1)
NIDDK, NHLBI: CLUES TO THE EFFICACY OF HBV TREATMENT WITH PEGYLATED INTERFERON-ALPHA
A team of NIH scientists set out to learn why many people with chronic hepatitis B virus (HBV) do not respond to current treatment with pegylated interferon alpha (PEG-IFN-a). In a pilot study, the researchers studied blood samples and liver biopsies isolated from 13 people with chronic HBV infection before, during, and after treatment with PEG-IFN-a. They found that people responded better to the treatment when their body’s natural killer cells (immune system cells that control viral infections) were activated. The researchers also found that people who developed more antibodies against PEG had less of a response, because these antibodies removed PEG-IFN-a from the blood. These findings may improve the efficacy of HBV treatment and may be applicable to similar drugs now being developed for other chronic diseases and cancer. (NIH authors: A. Nishio, F.J. Bolte, K. Takeda, N. Park, Z.-X. Yu, H. Park, K. Valdez, M.G. Ghany, and B. Rehermann, Sci Transl Med 13:Issue 587, eaba6322, 2021; DOI:10.1126/scitranslmed.aba6322)
This page was last updated on Thursday, February 3, 2022
