Research Briefs
NIEHS: DNA REPAIR DISCOVERY HOLDS PROMISE FOR PRECISION CANCER THERAPY
CREDIT: SHUTTERSTOCK
NIEHS: A new study highlights BRCA1-2 and APEX2 synthetic lethality, which means that the combined lack of both gene products is lethal to breast cancer cells. Shown: DNA strands.
NIEHS researchers and collaborators in Canada discovered that cancer cells with mutated BRCA1 and BRCA2 genes died when they lacked a protein called apurinic or apyrimidinic endonuclease (APE2), which promotes the repair of damaged DNA. APE2 is encoded by APEX2 gene. The mechanism behind BRCA1-2 and APEX2 synthetic lethality (when the combined lack of both gene products is lethal) to breast-cancer cells highlights a vulnerability that may lead to a precision-medicine approach to treating BRCA1-2–deficient breast cancers. (NIEHS authors: J.L. Wojtaszek, T. Patel, C.D. Appel, B.D. Wallace, and R.S. Williams, Mol Cell 78:1152–1165.E8, 2020; DOI:10.1016/j.molcel.2020.05.021)
NIA: GUM DISEASE LINKED WITH DEMENTIA
Periodontal (gum) disease has been linked with heart disease, preterm labor, and many other health conditions including Alzheimer Disease (AD). Lab studies have suggested that both bacteria and the toxins they produce can travel through the bloodstream to the brain, causing inflammation and other neurotoxicities. NIA researchers were the first, however, to conduct a large retrospective analysis of population data to examine the association between periodontal pathogens, as well as antibodies against certain oral pathogens, with the incidence and mortality of AD and other dementias.
The NIA research team used data from the National Health and Nutrition Examination Survey, Medicare, and the National Death Index to examine whether periodontal disease and antibodies against 19 bacteria might be linked to dementia diagnoses and deaths. The team compared different age groups at baseline with up to 26 years of follow-up for more than 6,000 participants. The analysis revealed that older adults with signs of periodontal disease and antibodies against oral pathogens at baseline were more likely to develop AD. Among those 65 years or older, both AD diagnoses and deaths were associated with antibodies against the oral bacterium Porphyromonas gingivalis with additional clustering of other bacteria species. Although the study provides evidence for an association between periodontal pathogens and AD, which was stronger for older adults, the authors called for further investigations, including randomized controlled trials, on the effectiveness of periodontal treatment against the onset and progression of neurodegenerative disorders such as AD. (NIA authors: M. Beydoun, S. Hossain, and A.B. Zonderman, J Alzheimers Dis 75:157–172, 2020; DOI:10.3233/JAD-200064.)
NIDDK, NIEHS, NIDA: THE LURE OF HIGH-FAT DIETS
The brain may be wired to drive the intake of high-fat foods, according to a study conducted in mice by an international team of scientists including researchers from NIDDK, NIEHS, and NIDA. The investigators found that after exposure to high-fat, calorie-rich foods, mice no longer wanted a nutritionally balanced, standard diet, even when they were hungry and no longer had access to the high-fat foods. The high-fat diet was the only way to “switch off” the neurons responsible for the unpleasant sensations linked to hunger.
The researchers also looked at the neurons associated with the sense of reward that comes with food discovery. They found that these neurons showed significantly decreased responses to the nutritionally balanced foods after the mice were exposed to the high-fat foods. Because the neurons the researchers studied in mice have nearly identical functions in people, these findings suggest a neural basis behind the challenges of human dieting. (NIH authors: C.M. Mazzone, J. Liang-Guallpa, C. Li, N.S. Wolcott, M.H. Boone, M. Southern, N.P. Kobzar, I. De Araujo Salgado, D.M. Reddy, G. Cui, and M.J. Krashes, Nat Neurosci 2020; DOI:10.1038/S41593-020-0684-9)
NHGRI, NCBI: COMPLETE HUMAN X CHROMOSOME SEQUENCE GENERATED
Researchers from NIH and other institutions have produced the first end-to-end DNA sequence of a human chromosome. Because a human genome is incredibly long, consisting of about 6 billion bases, DNA-sequencing machines cannot read all the bases at once. Instead, the genome is chopped into smaller pieces; each piece is analyzed to yield sequences of a few hundred bases at a time; and then these sequences are reassembled in much the same way that a jigsaw puzzle is reconstructed. The scientists chose to complete the X chromosome sequence and capitalized on new technologies that leave DNA molecules largely intact. These large DNA molecules were then analyzed by two different instruments, each of which generates very long DNA sequences–something previous instruments could not accomplish. Being able to generate a precise, base-by-base sequence of a human chromosome will enable researchers to produce a complete sequence of the human genome. (NIH authors: S. Koren, A. Rhie, S. Brooks, V.A. Schneider, E. Pak, V. Maduro, A. Dutra, G.G. Bouffard, A.M. Chang, N.F. Hansen, F. Thibaud-Nissen, A. Young, J.C. Mullikin, and A.M. Phillippy, Nature 585:79–84, 2020; DOI:10.1038/S41586-020-2547-7)
NINDS: TURNING OFF “JUNK DNA” MAY FREE STEM CELLS TO BECOME NEURONS

CREDIT: A. NATH LAB, NINDS
NIH scientists showed how ancient retroviral genes, or “junk DNA,” may play a role in helping stem cells decide to become neurons.
Over the course of evolution, the human genome has absorbed thousands of human endogenous retrovirus genes. As a result, nearly eight percent of the DNA that lines our chromosomes includes remnants of these genes. Although once thought to be inactive, or “junk,” recent studies have shown that these genes may be involved in human embryonic development, the growth of some tumors, and nerve damage during multiple sclerosis. Previously, NINDS researchers showed that amyotrophic lateral sclerosis may be linked to activation of the HERV-K gene. In the new study, the team showed that deactivation of the gene may free stem cells to become neurons. In the future, the team plans to explore how HERV-K genes may shape the wiring of a nervous system. (NINDS authors: T. Wang, M. Medynets, K.R. Johnson, T.T. Doucet-O’Hare, B. DiSanza, W. Li, Y. Xu, A. Bagnell, R. Tyagi, K. Sampson, N. Malik, J. Steiner, Alina Hadegan, J. Kowalak, J. O’Malley, D. Maric, and A. Nath, Proc Natl Acad Sci U S A 117:17842–17853, 2020; DOI:10.1073/pnas.2002427117)
NICHD: HOW TO STARVE A MALARIA PARASITE
CREDIT: NIAID
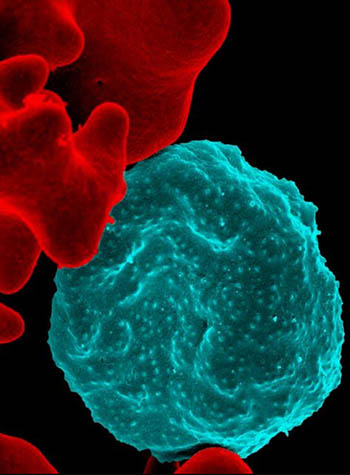
NICHD: A new study shows that starving malaria parasites may be one way to stop malaria. Shown: (Center, blue) Colorized scanning electron micrograph of red blood cell infected with malaria parasites; (left) uninfected cells with a smooth red surface.
In 2018, there were 228 million cases of malaria worldwide, leading to more than 400,000 deaths, 67% of which were among children under 5, according to the World Health Organization. One way to stop the infections would be to starve the malaria parasite, Plasmodium falciparum, once it infects a person but before it can do any damage. NIH researchers are making discoveries about how the parasite gets its nutrients as it infects red blood cells. In 2000, NIH researchers found a set of porelike holes in the membranes of red blood cells. The parasite uses those holes to supply itself with non-fatty nutrients. In a recent study, NIH researchers discovered another set of channels—ones that enable the transport of fatty nutrients between the blood cell and the parasite. Together the discoveries may lead to treatments that would starve the parasite and keep it from proliferating in the body. (NIH authors: M. Garten, T. Tenkova-Heuser, J. Heuser, and J. Zimmerberg, Nat Commun 11:article number3825, 2020; DOI:10.1038/s41467-020-17506-9)
NIAMS, NCI: REDUCTIONS IN SMOKING AND HEAVY DRINKING MAY MEAN FEWER HIP FRACTURES
A new study, which analyzed 40 years of Framingham Heart Study data, found an association between lowered rates of hip fractures and decreases in smoking and heavy drinking. The rates of hip fractures in the United States have been declining over the past few decades. Although some experts attribute this change primarily to improved treatments for bone health, a new study conducted by researchers from NIH and other institutions suggests other factors. These results indicate that modifiable lifestyle factors, along with treatments, may be beneficial to bone health.
The analysis included information from 4,918 men and 5,634 women who were followed for a first hip fracture between 1970 and 2010. The rates for hip fractures, which were adjusted for age, dropped by 4.4% each in both men and women. In addition, the rate of smoking decreased from 38% in the 1970s to 15% in the period from 2006 to 2010. During the same period, heavy drinking (defined as three or more drinks per day) fell from 7% to 4.5%. The rates of other risk factors for hip fracture, such as underweight and early menopause, did not change over the study period. The study authors noted that because the data were exclusively from white individuals, it is unclear whether other populations might show a similar correlation based on lifestyle factors. Another limiting factor was that Framingham participants had lower rates of obesity than the national average. Additionally, the study did not include measurements of bone mineral density, because such testing was not available until the 1990s. (NIH authors: J. Swayambunathan, A. Dasgupta, P.S. Rosenberg, and T. Bhattacharyya, JAMA Intern Med 2020; DOI:10.1001/jamainternmed.2020.2975)
NEI: DUAL ROLE DISCOVERED FOR MOLECULE INVOLVED IN AUTOIMMUNE EYE DISEASE
CREDIT: RACHEL CASPI, NEI
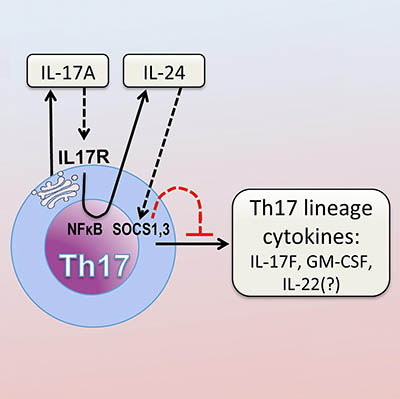
After activation through its T-cell receptor, Th17 produce IL-17A, which binds to its own receptor on the Th17 cell. This activates the NFκB pathway. NFκB drives production of IL-24, which in turn suppresses the Th17 cytokine program via SOCS1 and 3.
The inflammatory molecule interleukin-17A (IL-17A) triggers immune cells that in turn reduce IL-17A’s pro-inflammatory activity, according to a study by NEI researchers. In models of autoimmune diseases of the eye and brain, blocking IL-17A increased the presence of other inflammatory molecules produced by Th17 cells, immune cells that produce IL-17A and are involved in neuroinflammation. The finding could explain why IL-17-targeted treatments for conditions like the eye disease autoimmune uveitis and multiple sclerosis have failed. (NIH authors: W.P. Chong, M.J. Mattapallil, K. Raychaudhuri, S.-J. Bing, P.B. Silver, Y. Jittayasothorn, C.-C. Chan, R. Horai, and R.R. Caspi, Immunity 53:384–397.e5, 2020; DOI:10.1016/j.immuni.2020.06.022)
NIMH: SEX DIFFERENCES IN HUMAN BRAIN
A scientific analysis of more than 2,000 brain scans found evidence for highly reproducible sex differences in the volume of certain regions in the human brain, according to an NIMH-led study. This pattern of sex-based differences in brain volume corresponds with patterns of sex-chromosome gene expression observed in postmortem samples from the brain’s cortex, suggesting that sex chromosomes may play a role in the development or maintenance of sex differences in brain anatomy.
To explore the neurobiological basis of sex differences in the human brain, the team first analyzed neuroimaging data collected as part of the Human Connectome Project. The data, obtained from 976 healthy adults between the ages of 22 and 35, revealed consistent sex differences in the volume of certain cortical structures. On average, females had relatively greater cortical volume in the medial and lateral prefrontal cortex, orbitofrontal cortex, superior temporal cortex, and lateral parietal cortex. Males, on average, had relatively greater cortical volume in ventral temporal regions and occipital regions, including the temporal pole, fusiform gyrus, and primary visual cortex. Specifically, regions of the cortex with relatively high expression of sex-chromosome genes tended to have greater cortical volume in males than females.
The findings are consistent with results from earlier mouse studies, suggesting that sex differences in brain anatomy may be due, at least in part, to genetic mechanisms that have been conserved throughout mammalian evolution. Sex differences in brain organization are theoretically important for our understanding of sex differences in human cognition and behavior, according to the authors. (NIH authors: S. Liu, J. Seidlitz, J.D. Blumenthal, L.S. Clasen, and A. Raznahan, Proc Natl Acad Sci U S A 117:18788–18798, 2020; DOI:10.1073/pnas.1919091117)
This page was last updated on Tuesday, March 22, 2022
