COVID-19 Timeline at NIH (July–August 2020)
COVID-19 Research and Activities at NIH
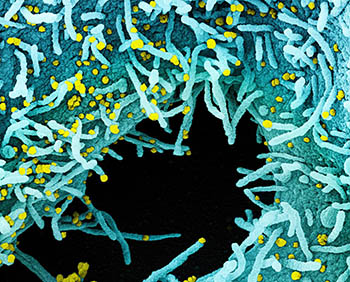
CREDIT: NIAID INTEGRATED RESEARCH FACILITY
Colorized scanning electron micrograph of a cell heavily infected with SARS-CoV-2 virus particles (yellow), isolated from a patient sample. The black area in the image is extracellular space between the cells. Image captured at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
July: Since May 21, the NIH Clinical Center has been performing weekly, voluntary testing to screen for SARS-CoV-2 infection in asymptomatic clinical staff approved to work on site in Building 10. In July, the Clinical Center expanded access to this testing to nonclinical staff approved to work on site in Building 10. Other NIH locations in Baltimore and Frederick, Maryland, North Carolina, Massachusetts, and Arizona also have begun conducting asymptomatic testing of approved onsite staff; the samples are sent to the Clinical Center for analysis.
July 1: In a “Perspective” for the New England Journal of Medicine, members of NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) Vaccines Working Group assess practical considerations and prerequisites for using controlled human infection models (CHIMs), which can be used for human challenge studies, to support SARS-CoV-2 vaccine development. In the article, the authors determine the timeline for developing robust CHIMs that meet the essential criteria for limiting risk for study volunteers, and the process could take one to two years. The authors conclude that large, randomized, controlled trials of SARS-CoV-2 are the fastest and most effective path forward for establishing vaccine safety and efficacy. (N Engl J Med 383:e63, 2020; DOI:10.1056/NEJMp2020076)
July 2: NIH Director Francis Collins, in testifying before the Senate Appropriations Subcommittee on Labor, Health and Human Services, Education, and Related Agencies, estimates that $18.8 billion in emergency funding would be needed for the NIH to respond to COVID-19. That includes approximately $10 billion to offset expenses associated with lab disruptions. He described the financial difficulties faced by universities and medical centers because of the shuttered labs in addition to reduced income from elective surgeries and other medical procedures.
July 8: NIAID announces the launch of a new clinical-trials network called the COVID-19 Prevention Trials Network. The network aims to enroll thousands of volunteers in large-scale trials testing a variety of investigational vaccines and monoclonal antibodies (mAbs) intended to protect people from COVID-19. In less than 24 hours, more than 45,000 people registered as potential volunteers!
July 10: In his all-staff email, NIH Director Francis Collins announces that NIAID is enrolling NIH staff who have recovered from COVID-19, and have been cleared to return to work, in the NIAID Antibody Research Study.
July 14: A study by NICHD and outside investigators finds the placental membranes that contain the fetus and amniotic fluid lack the messenger RNA molecule required to manufacture the angiotensin-converting enzyme 2 receptor, the main cell-surface receptor used by the SARS-CoV-2 virus to cause infection. (eLife 9:e58716, 2020; DOI:10.7554/eLife.58716)
July 14: An mRNA-1273 investigational vaccine, co-developed by researchers at Moderna, Inc., and NIAID’s Vaccine Research Center (VRC), was generally well tolerated and prompted gratifying titers of neutralizing antibodies in healthy adults in a NIAID-supported phase 1 trial. Plans are underway to launch a phase 3 efficacy trial for this vaccine in just a couple of weeks. The experimental vaccine is designed to induce neutralizing antibodies directed at a portion of the coronavirus “spike” protein, which the virus uses to bind to and enter human cells. (N Engl J Med 2020; DOI:10.1056/NEJMoa2022483)
July 14: The “NIH Workforce COVID-19 Impact Survey” for all staff including employees, contractors, students, fellows, and volunteers is launched to help assess the impact of COVID-19 on the NIH workforce.
July 20: Group B begins coming back to the workplace.
July 22: Several NIH institute and center directors and NIH Director Francis Collins outlined the efforts of the Rapid Acceleration of Diagnostics (RADx) initiative in a special report in the New England Journal of Medicine. They describe the current testing landscape for SARS-CoV-2, explained the urgent need for nationwide deployment of low-complexity, point-of-care molecular diagnostics with rapid results, and described the critical need to ensure testing is available to diverse, vulnerable, and underserved populations, which are disproportionately affected by the virus. (N Engl J Med 2020; DOI:10.1056/NEJMsr2022263)
July 27: A phase 3 clinical trial designed to evaluate whether an investigational vaccine can prevent symptomatic coronavirus disease 2019 (COVID-19) in adults begins. The vaccine, known as mRNA-1273, was co-developed by NIAID and Moderna, Inc. The trial, which will be conducted at U.S. clinical-research sites, is expected to enroll approximately 30,000 adult volunteers who do not have COVID-19.
July 27: NIH Director Francis Collins’s email to employees shares the news that Pfizer and BioNTech announced the launch of their combined phase 2/3 vaccine trial in the United States for their experimental coronavirus vaccine.
July 28: Two doses of an experimental vaccine to prevent COVID-19 induced robust immune responses and rapidly controlled the coronavirus in the upper and lower airways of rhesus macaques (Macaca mulatta) exposed to SARS-CoV-2, the virus that causes COVID-19. (N Engl J Med 2020; DOI: 10.1056/NEJMoa2024671)
July 30: Group B staff at the Baltimore County, Maryland, locations begin returning to their physical workspaces.
July 31: NIH announces that it is investing $248.7 million in new technologies to address challenges associated with COVID-19 testing. NIH’s RADx initiative has awarded contracts to seven biomedical diagnostic companies to support a range of new lab-based and point-of-care tests that could significantly increase the number, type, and availability of tests by millions per week as early as September 2020. With national demand estimated to be millions more tests per day above current levels, these technologies are expected to make a significant contribution to expanding the nation’s testing capacity.
July 31: NIAID Director Anthony Fauci and other health experts testify before the House Oversight and Reform Select Subcommittee on the Coronavirus Crisis. He says he is “cautiously optimistic” the United States could have a safe and effective vaccine by the “end of this year and as we go into 2021.” He tells lawmakers that the vaccine may not be available to Americans immediately, but in phases. He also says the NIH’s strategic plan is focused on addressing four key points related to COVID-19: the improvement of fundamental knowledge of the virus; the development of diagnostics; the testing of therapeutics; and development and testing of vaccines.
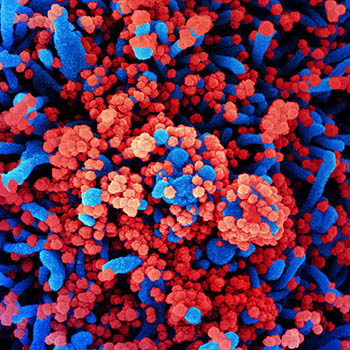
CREDIT: NIAID
Colorized scanning electron micrograph of a cell (blue) heavily infected with SARS-CoV-2 virus particles (red), isolated from a patient sample.
August 4: NIH launches a clinical trial to test mAb treatment in hospitalized COVID-19 patients at select hospitals. The phase 3 randomized, controlled trial, known as ACTIV-3, is designed to expand to test multiple different kinds of mAb treatments. The new study is one of four ongoing or planned trials in NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, a public-private partnership to speed development of the most promising treatments and vaccine candidates. It also is receiving support through Operation Warp Speed, the U.S. government’s multiagency effort to develop, manufacture, and distribute medical countermeasures to fight COVID-19.
August 4: NIH-supported researchers launch a phase 2 clinical trial for outpatients with mild and moderate COVID-19 to test whether mAbs and other experimental therapeutics can reduce the severity of symptom. The first therapeutic to be tested in this trial will be LY-CoV555, an investigational mAb made by Eli Lilly and Company. The antibody emerged from Lilly’s collaboration with AbCellera Biologics, which, together with NIAID’s VRC, discovered the antibody when it was isolated from a blood sample from a recovered COVID-19 patient.
August 5: The NIH-Moderna investigational COVID-19 vaccine, mRNA-1273, protected mice from infection with SARS-CoV-2, the virus that causes COVID-19, according to research published in Nature. Scientists at NIAID, Moderna, and other institutions conducted the preclinical research. NIAID VRC scientists worked with investigators from the University of Texas at Austin to identify the atomic structure of the spike protein on the surface of the novel coronavirus. This structure was used by VRC and Moderna in the development of the vaccine candidate. (Nature 2020; DOI:10.1038/s41586-020-2622-0)
August 5: NIH launches the Medical Imaging and Data Resource Center (MIDRC), an ambitious effort that will harness the power of artificial intelligence and medical imaging to fight COVID-19. The multi-institutional collaboration, led by NIBIB, will create new tools that physicians can use for early detection and personalized therapies for COVID-19 patients. The MIDRC will facilitate rapid and flexible collection, analysis, and dissemination of imaging and associated clinical data.
August 6: An NIAID-sponsored clinical trial testing the antiviral remdesivir plus the immunomodulator interferon beta-1a for COVID-19 treatment begins. The study, called the Adaptive COVID-19 Treatment Trial , is anticipated to enroll more than 1,000 hospitalized adults with COVID-19 at as many as 100 sites in the United States and abroad.
August 10: Two phase 3, randomized, placebo-controlled, double-blind clinical trials testing whether experimental mAbs can prevent infection by SARS-CoV-2 coronavirus are now enrolling healthy adults at clinical-trial sites in the United States. The trials are enrolling adults who are at risk of infection due to close contact at work or home to persons with SARS-CoV-2 infection. One trial, being conducted jointly by NIAID and trial sponsor Regeneron Pharmaceuticals, will evaluate Regeneron’s investigational double mAb combination, REGN-COV-2, which is designed to bind to two points on the SARS-CoV-2 spike protein and prevent it from entering healthy cells. The other trial, sponsored by Eli Lilly and Company and implemented in collaboration with NIAID, will evaluate LY-CoV555, isolated from a recovered COVID-19 patient by scientists at AbCellera and the NIAID VRC, and developed by Eli Lilly and Company.
August 10: An email to DC area staff announces that the NIH Clinical Center has begun testing asymptomatic staff for SARS-CoV-2 in Groups 0, A, and B working on site. The program is voluntary but strongly encouraged for those working on site. Other NIH locations in Baltimore, Maryland, North Carolina, and Montana also have begun conducting asymptomatic testing of approved on-site staff, with all lab analyses done at the NIH Clinical Center.
August 21: New FAQ section on child care is added to the NIH intramural site for coronavirus guidance.
Aug 26: The Clinical Center announces that asymptomatic testing is available for all DC-area staff and that appointments are needed.
August 28: The COVID-19 pandemic has taken a disproportionate toll on people with intellectual and developmental disabilities, write the directors of the Intellectual and Developmental Disabilities Research Centers network, a nationwide group funded by NICHD, in the American Journal of Psychiatry. (Am J Psychiatry 2020; appiajp202020060780; DOE:10.1176/appi.ajp.2020.20060780)
Aug 31: Staff approved to work on site (Groups 0, A, and B) will no longer receive a message from Alert NIH to self-assess for COVID-19 and will instead start using the SAFER-COVID tool to complete a brief daily self-assessment. (Instructional video at https://www.youtube.com/watch?v=Im-hH4k_WHs)
August 31: Phase 3 clinical testing of the AstraZeneca investigational COVID-19 vaccine known as AZD1222 begins. The trial will enroll approximately 30,000 adult volunteers at 80 sites in the United States to evaluate whether the candidate vaccine can prevent symptomatic COVID-19. AstraZeneca is leading the trial as regulatory sponsor; NIAID and the Biomedical Advanced Research and Development Authority (part of HHS) are providing funding support. Scientists at NIAID’s Rocky Mountain Laboratories, based in Hamilton, Montana, conducted a preclinical study of AZD1222.
This page was last updated on Tuesday, March 22, 2022
