Research Briefs: COVID-19
NIAID: REPURPOSED VACCINE PROTECTS AGAINST COVID-19 PNEUMONIA IN ANIMALS
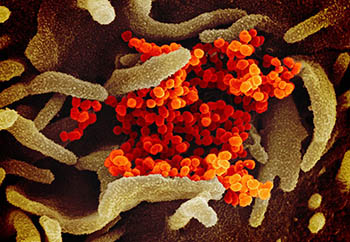
CREDIT: ROCKY MOUNTAIN LABS, NIAID
This scanning electron microscope image shows SARS-CoV-2 (orange), the virus that causes COVID-19, isolated from a patient in the United States. The virus (red) is emerging from the surface of cells (green) cultured in the lab.
In a joint effort between NIAID’s Rocky Mountain Laboratories (RML) in Hamilton, Montana, and the University of Oxford’s Jenner Institute in Oxford, England, researchers have shown that a single dose of the investigational vaccine ChAdOx1 nCoV-19 elicits a protective immune response against SARS-CoV-2 pneumonia in monkeys. SARS-CoV-2 is the virus that causes COVID-19. The findings, first published in the preprint server bioRxiv (and later in Nature), have allowed for the launch of a phase 1 clinical trial in humans.
By adapting an existing adeno-vaccine platform (ChAdOx1), previously used to protect nonhuman primates against MERS-CoV-induced disease–another infamous coronavirus–the team from England replaced the sequence encoding the MERS spike protein with the sequence encoding that of SARS-CoV-2.
The RML team showed that the vaccine elicited a significant immune response, compared with control animals, in both mice and rhesus macaques (Macaca mulatta). There was no sign of virus replication in the lungs; significantly lower amounts of respiratory disease; no damage to lung tissue; no evidence of immune-enhanced disease; and development of spike-specific antibodies to SARS-CoV-2. (NIH authors: N. van Doremalen, J.N. Purushotham, J.R. Port, V. Avanzato, T. Bushmaker, F. Feldmann, J. Schulz, M. Holbrook, A. Okumura, K. Meade-White, L. Pérez-Pérez, B.N. Williamson, R. Rosenke, D. Long, J. Lovaglio, P.W. Hanley, D. Scott, G. Saturday, E. de Wit, and V.J. Munster, Nature, 2020; DOI:10.1038/s41586-020-2608-y)
NIAID: REMDESIVIR IMPROVES COVID-19 RECOVERY TIME
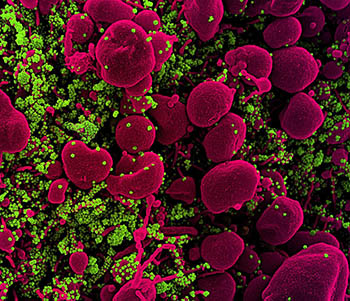
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (pink) heavily infected with SARS-COV-2 virus particles (green) isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
The investigational antiviral remdesivir is superior to the standard of care for the treatment of COVID-19, according to a study by NIAID researchers and international partners. In an Adaptive COVID-19 Treatment Trial (ACTT), the researchers compared remdesivir to a placebo in a phase 3, randomized, double-blind, placebo-controlled clinical trial that enrolled 1,063 participants in 10 countries in 58 days. The participants were hospitalized adults with laboratory-confirmed COVID-19 and lower respiratory tract involvement.
Patients provided informed consent to participate in the trial and were randomly assigned to receive local standard care and a 10-day course of remdesivir intravenously or local standard care and a placebo. After preliminary analysis, the researchers found that remdesivir was superior to the placebo in shortening the time to recovery. Hospitalized patients with severe disease who needed supplemental oxygen benefitted the most from remdesivir. The authors emphasized that starting antiviral treatments early in the disease is necessary to prevent COVID-19 from progressing to the point that infected patients require mechanical ventilation. As the investigators expressed a need for assessing antivirals combined other therapeutic agents, NIAID began the ACTT-2 clinical trial to evaluate remdesivir with baricitinib, an anti-inflammatory drug. (NIH authors: J.H. Beigel, K.M. Tomashek, L.E. Dodd, T. H. Burgess, T. Bonnet, M. Green, M. Makowski, S. Nayak, and H.C. Lane; New Eng J Med, 2020; DOI:10.1056/NEJMoa2007764)
OD: ACTIV AND COLLABORATIVE RESPONSES IN THE TIMES OF COVID-19
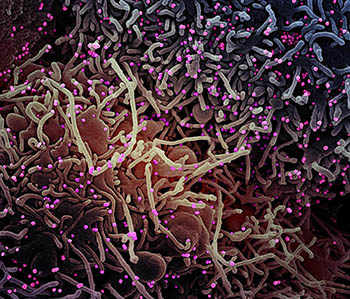
CREDIT: NIAID
Colorized scanning electron micrograph of a VERO E6 cell (purple) exhibiting elongated cell projections and signs of apoptosis, after infection with SARS-CoV-2 virus particles (pink), which were isolated from a patient sample. Image captured at the NIAID Integrated Research Facility in Fort Detrick, Maryland.
Facing the COVID-19 pandemic, NIH Director Francis Collins and a Johnson & Johnson official called for rapid, collaborative response efforts in a recent viewpoint article published in the Journal of the American Medical Association. The authors outlined the response in the NIH-led Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) initiative, an unprecedented private-public partnership among United States federal agencies, the European Medicines Agency, and biopharmaceutical companies.
ACTIV brings together the public and private sectors in a never-before-seen coordinated strategy for an integrated international research response to COVID-19. The goals of ACTIV, the authors say, are to establish a collaborative framework for prioritizing therapeutic and vaccine candidates, to streamline clinical trials and use existing trial networks, and to coordinate regulatory processes to expedite scale up and manufacturing of therapeutics and vaccines. ACTIV has created four working groups to accomplish these goals, each with an industry and NIH cochair: Preclinical Group, Therapeutics Clinical Group, Clinical Trial Capacity Group, and Vaccines Group. Industry partners within the working groups pledge to support promising therapeutic and vaccine candidates and to contribute clinical-trial capacities, irrespective of who is developing the candidates and what agent is under development. The authors point out that ACTIV is the first harmonized public-private biomedical research effort of this global scope and scale. (NIH author: F.S. Collins, JAMA 2020; DOI:10.1001/jama.2020.8920)
OD, NIAID, VRC: A STRATEGIC APPROACH FOR DEVELOPING COVID-19 VACCINES
During the global mobilization to develop a COVID-19 vaccine, NIH Director Francis Collins and NIAID scientists called for a harmonized approach to clinical testing, scale-up, and distribution of vaccine candidates in a recent Science perspective article. To provide blanket protection from COVID-19, the scientists stated that no single vaccine or platform is likely to meet the global need; they called for strategic research and development approaches to generate data in parallel for multiple vaccine candidates.
The authors identified key considerations for diverse vaccine candidates, such as prior commercial experience, scalability, and the types of immune responses generated by various vaccine platforms. They emphasized that, because currently little is known about COVID-19 and immunity, researchers need to determine what constitutes a protective immune response. For vaccine efficacy trials, the authors say that multiple candidates can be evaluated in parallel to generate essential safety and efficacy data. Doing so would increase the speed of vaccine distribution and licensing. They indicated that running parallel trials needs coordinated clinical testing with master protocols, common clinical-trial designs, common clinical endpoints, and a data and safety monitoring board. Successful COVID-19 vaccine development, the authors said, calls for unprecedented collaboration among governments, academic institutions, biopharmaceutical corporations, and philanthropic partners. (NIH authors: J.R. Mascola, A.S. Fauci, and F.S. Collins, Science 368:948–950, 2020; DOI:10.1126/science.abc5312)
NLM: KEY GENOMIC FEATURES THAT COULD DIFFERENTIATE SARS-COV-2 FROM OTHER CORONAVIRUSES
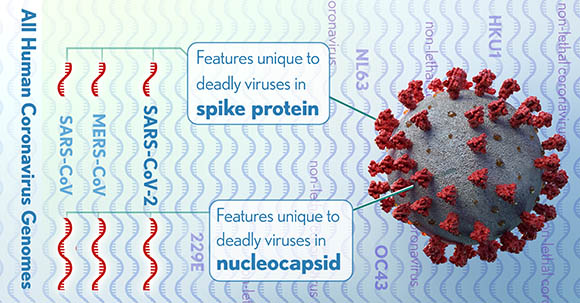
CREDIT: DONNY BLISS, NLM
The full genomes of all human coronaviruses were aligned to identify regions (red) that might code for lethal differences in the virus that causes COVID-19 as well as SARS and MERS. These differences could be targets for testing or treatments.
A team of researchers in NLM’s National Center for Biotechnology Information (NCBI) identified genomic features of SARS-CoV-2, the virus that causes COVID-19, and two other deadly coronaviruses, SARS-CoV and MERS CoV, that distinguish them from other members of the coronavirus family.
“We were able to identify several features that are not found in less virulent coronaviruses and that could be relevant for pathogenicity in humans,” said NCBI Distinguished Investigator Eugene Koonin, lead author of the study. “The actual relevance of these findings will come from direct experiments that are currently getting underway.”
The unique features predicted by protein-structure analysis include insertions of specific stretches of amino acids into two virus proteins, the nucleocapsid and the spike. These features are found in all three high-fatality coronaviruses and their closest relatives that infect animals, such as bats, but not in four other human coronaviruses that are endemic and cause mild symptoms (such as the common cold). The identified features in animal coronavirus isolates could predict the jump to humans and the severity of disease. Predictions made through this analysis may help identify possible targets for diagnostics and interventions. (NLM authors: A.B. Gussow, N. Auslander, Y.I. Wolf, and E.V. Koonin, Proc Natl Acad Sci USA 2020; DOI:10.1073/pnas.2008176117)
NCI-CCR, NIAID: POTENTIAL TREATMENT FOR RESPIRATORY DISTRESS IN SEVERE COVID-19
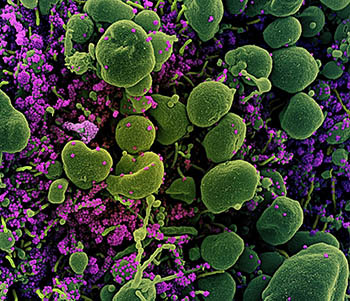
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-CoV-2 virus particles (purple), isolated from a patient sample.
Some patients with severe COVID-19 progress to a hyperinflammatory phase of illness for which there are no proven treatments. NIH scientists and colleagues at other institutions identified the Bruton tyrosine kinase (BTK) protein as a key instigator in this overactive immune response.
BTK plays a role in signaling macrophages (innate immune cells) to release cytokines, inflammatory proteins that normally coordinate the immune response. Researchers found that some COVID-19 patients in acute respiratory distress show evidence of hyperactive BTK, high blood concentrations of cytokines, and low lymphocyte (white blood cell) count. Termed a “cytokine storm,” this potentially fatal virus-induced state can lead to organ damage and compromise lung function.
In a prospective off-label clinical study, the researchers used the drug acalabrutinib to treat 19 hospitalized COVID-19 patients (11 on supplemental oxygen and eight on ventilators) with severe hypoxia and evidence of inflammation. Acalabrutinib is a BTK inhibitor currently approved to treat severe blood cancers. After the experimental treatment, most of the patients experienced improved respiratory function, and their inflammation and lymphocyte concentrations normalized. After 10 to 14 days, 73% of those on supplemental oxygen and 25% of those needing mechanical ventilation were discharged to room air.
“These results suggest that targeting excessive host inflammation with a BTK inhibitor is a therapeutic strategy in severe COVID-19 and has led to a confirmatory international prospective randomized controlled clinical trial,” the authors wrote. (NIH authors: M. Roschewski, M.S. Lionakis, J.V. Desai, M.A. Zarakas, G. Wright, L.M. Staudt, and W. Wilson, Sci Immunol 5:eabd0110, 2020; DOI:10.1126/sciimmunol.abd0110O)
NIAID, NCI: STEPS IDENTIFIED TO EXPAND AND IMPROVE ANTIBODY TESTS IN COVID-19 RESPONSE
More than 300 scientists and clinicians from the federal government (including NIH), industry, and academia who gathered for an online workshop in May, and, in June, published a report of their conclusions and recommendations on COVID-19 serology studies online in Immunity. Serology studies can indicate prior infection with SARS-CoV-2 that may have been missed because a person did not experience significant symptoms or access testing while infected. Workshop attendees recommended that additional research is needed to determine if and to what extent a positive antibody test means a person may be protected from reinfection with SARS-CoV-2. Until such data is available, serology tests should not be used as a stand-alone tool to make decisions about personal safety related to SARS-CoV-2 exposure. Researchers are now pursuing studies in humans and in animal models to better understand SARS-CoV-2 immunity. Workshop attendees noted that such understanding could help identify optimal donors of convalescent plasma that potentially could be used to help treat those with severe COVID-19. Attendees also proposed strategies to expand the accuracy and capacity of these tests to distinguish between naturally acquired and vaccine-induced antibodies, which will be critical to evaluating COVID-19 vaccine candidates. (NIH authors: A.M. Lerner, R.W. Eisingerr, D.R. Lowy, and M.C. Cassetti, Immunity, 2020; DOI: 10.1016/j.immuni.2020.06.012)
NIAID (RML): CD47 STUDY MAY LEAD TO BROAD–SPECTRUM INFECTIOUS DISEASES IMMUNOTHERAPY
NIH investigators and colleagues have discovered that when the immune system first responds to infectious agents such as viruses or bacteria, a natural brake on the response prevents overactivation. Their new study describes this brake and the way pathogens such as SARS-CoV-2, the virus that causes COVID-19, turn it on. Their finding provides a potential target for an immunotherapy that might be applied to a wide range of infectious diseases.
When a cell senses an infectious agent with molecules called pathogen-recognition receptors, part of its response is to increase cell-surface expression of a molecule called CD47, otherwise known as the “don’t eat me” signal. Increased CD47 expression dampens the ability of macrophages to engulf infected cells and further stimulate the immune response. Upregulation of CD47 on cells was observed for diverse types of infections including those caused by mouse retroviruses, lymphocytic choriomeningitis virus, LaCrosse virus, SARS CoV-2, and by the bacteria Borrelia burgdorferi and Salmonella enterica typhi. By blocking CD47-mediated signaling with antibodies in mice infected with lymphocytic choriomeningitis virus, the authors demonstrated they could enhance the speed of pathogen clearance. Furthermore, knocking out the CD47 gene in mice improved their ability to control M. tuberculosis infections and significantly prolonged their survival. In addition, retrospective studies of cells and plasma from people infected with hepatitis C virus indicated that humans also upregulate CD47. In these studies, inflammatory cytokine stimuli and direct infection both promoted increased CD47 expression. (NIH authors: L. Myers, K.D. Mayer-Barber, A.C. Bohrer, E. Castro, A.B. Carmody, R.J. Messer, M. Hasenkrug, Karin E. Peterson, C.W. Winkler, T.A. Woods, and K.J. Hasenkrug, mBio, 2020; DOI:10.1128/mBio.01293-20
This page was last updated on Wednesday, March 23, 2022
