Research Briefs
NINDS: NOSY VIRUSES THWARTED BY BRAINY IMMUNE CELLS
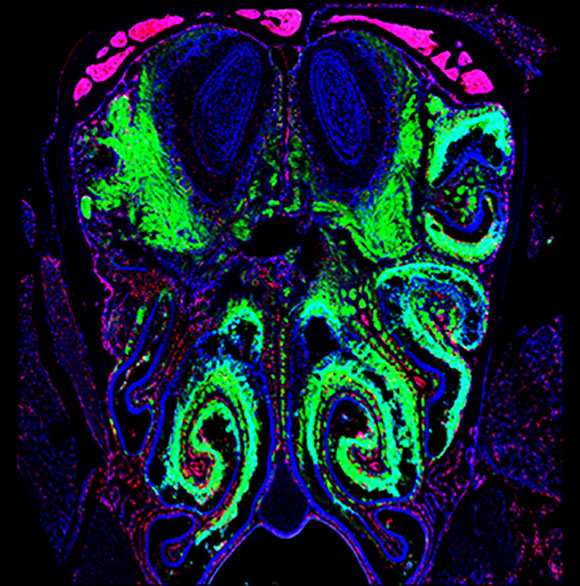
CREDIT: MCGAVERN LAB, NINDS
The olfactory bulb in the brain is the battleground where viruses (labeled in green) in the nasal passages are stopped from infecting the central nervous system (blue oval structures at the top of the image).
NINDS researchers discovered how the immune system protects our brains from infectious agents such as airborne viruses that can enter through the nose. The sensory neurons embedded in the lining of our nasal passages give us our sense of smell. These neurons create a conduit to the brain’s scent-processing center, the olfactory bulb, posing the risk of airborne pathogens hitching a ride directly to the central nervous system (CNS).
The researchers did experiments in mice and used a fluorescent-tagged vesicular stomatitis virus to see the path a virus takes from the nose to the olfactory bulb. The authors found a collaborative immune-cell response that mitigates damage to CNS neurons. Unexpectedly, microglia (immune cells within the CNS) face off with the infected sensory neurons in the olfactory bulb and sound the alarm for T cells to respond to pinpoint and destroy the infected cells before they can damage the brain; the microglia remain uninfected themselves.
Understanding this mechanism shines a new light on the significance of microglia in our brain’s defense and underscores how their depletion or diminished capacity could open the door for a virus to enter the brain and cause encephalitis or meningitis. Although the virus that causes COVID-19 was not studied in these experiments, some of the symptoms it produces suggest that the same mechanism described here could be in play, according to NINDS senior investigator Dorian McGavern, who led the study. (NIH authors: E.A. Moseman, A.C Blanchard, and D.B. McGavern, Sci Immunol 5:eabb1817, 2020; DOI:10.1126/sciimmunol.abb1817)
NCI-CCR, CC: EFFECTIVE, LESS TOXIC ALTERNATIVE TO STANDARD TREATMENT FOR ADULTS WITH BURKITT LYMPHOMA
A multicenter study led by NIH researchers found that an alternative treatment regimen was highly effective for adults with Burkitt lymphoma across all age groups and independent of HIV status. Burkitt lymphoma is a rare but aggressive B-cell lymphoma that is more common in children than adults. Although the standard dose-intensive chemotherapy (which uses very high doses) can cure the disease, this treatment can be acutely toxic for adults, especially those who are older or who are living with HIV/AIDS. The authors suggest, however, that dose-adjusted (DA) EPOCH-R, which is already used to treat people with diffuse large B-cell lymphomas, is a potentially curative treatment option for elderly patients and those with HIV and other co-morbidities, who might not be able to receive standard treatment.
In an earlier pilot study with 30 adult patients treated only at NCI, the DA-EPOCH-R regimen was found to be effective. This regimen is tailored to an individual patient’s ability to tolerate chemotherapy. To confirm these findings, the researchers conducted a larger, multicenter phase 2 study with 113 patients who had low- or high-risk disease. The treatment was effective across all age groups, including patients in their 70s and 80s, and regardless of HIV status. Furthermore, EPOCH-R can be administered in an outpatient setting, an alternative to the prolonged hospitalization required to receive highly dose-intensive chemotherapy. The researchers found that patients who had disease in the cerebrospinal fluid (CSF) had the highest risk of treatment-related death or treatment failure; there were five treatment-related deaths in the study. Further studies are warranted to determine how best to treat patients with CSF involvement. (NIH authors: M. Roschewski, C. Melani, A.N. Lucas, S.M. Steinberg, S. Pittaluga, E.S. Jaffe, R.F. Little, and W.H. Wilson, J of Clin Oncol 2020; DOI:10.1200/JCO.20.00303)
NINR, NINDS: BIOMARKERS MAY PREDICT RISK OF COMPLICATIONS AFTER MILD TBI
NIH scientists have brought us closer to understanding a neuroinflammatory connection between mild traumatic brain injury (mTBI) and chronic conditions such as post-traumatic stress syndrome (PTSD), postconcussive syndrome (PCS), and depression. In a recent study, investigators at NINR, NINDS, and other institutions identified predictive blood biomarkers in veterans with a history of repetitive mTBI.
Following injury to neural tissue, indicators of cellular damage are released into the blood and exosomes (extracellular vesicles carrying proteins and other molecules). This study analyzed plasma and exosomal concentrations of neurofilament light chain, a structural protein found in neurons, and inflammatory markers such as tumor necrosis factor (TNF)–alpha, interleukin (IL)–6, IL-10, and vascular endothelial growth factor. Researchers then compared biomarker concentrations in a cohort of 195 military veterans enrolled in the Chronic Effects of Neurotrauma Consortium Longitudinal Study among those with no history of TBI (control subjects), those with one to two mTBIs, and those more than two mTBIs.
Elevated neurofilament light chain was associated with PCS, PTSD, and depression symptoms; increased TNF–alpha was also observed in those with PCS and PTSD. The researchers found a significant correlation between higher biomarker concentrations in veterans with a history of repetitive mTBIs, with length of time in years since the last mTBI, and with increased severity of neurological and behavioral symptoms. “Our findings demonstrate the potential of these easily accessible biomarkers as predictors of behavioral alterations in veterans years after mTBI and provide insights into potential underlying pathologic mechanisms,” the authors wrote. (NIH authors: V.A. Guedes, K. Kenney, P. Shahim, B. Qu, C. Lai, C. Devoto, and J.M. Gill, Neurology 94:e2412-e2423, 2020; DOI 10.1212/WNL.0000000000009577)
NIA, NIAAA: REPURPOSED DRUG HELPS OBESE MICE LOSE WEIGHT
Obesity is a major public health challenge and has reached epidemic proportions in developed countries. A study conducted by NIA and NIAAA researchers and others showed that disulfiram, an FDA-approved drug for treating chronic alcohol addiction, normalized body weight, restored insulin responsiveness, and improved liver and pancreas functions in obese middle-aged mice of both sexes.
The study was performed on groups of 9-month-old lab mice who had been fed a high-fat diet for 12 weeks, causing them to become overweight and display signs of prediabetes metabolic problems such as insulin resistance and elevated fasting blood-glucose concentrations. These mice were then divided into four groups and fed four different diets for 12 more weeks: a standard diet alone; a high-fat diet alone; a high-fat diet with a low dose of disulfiram; or a high-fat diet with a higher dose of disulfiram. As expected, mice fed a standard diet normalized their body weight, fat composition, and blood glucose while the mice fed a high-fat diet alone continued to gain weight and show metabolic irregularities. Interestingly, mice fed a high-fat diet with either the low- or the high-dose disulfiram also normalized their body weights, insulin responsiveness, and blood-glucose concentrations. The disulfiram treatment also seemed to protect the liver and pancreas from damage.
The authors cautioned that the results are based on animal studies, and it is not known whether disulfiram would help people with morbid obesity lose weight. The researchers are planning future studies, including a controlled clinical trial, to determine the drug’s potential. (NIH authors: M. Bernier, S.J. Mitchell, D. Wahl, W. Seo, M. Wang, A. Ali, T. Kaiser, M.A. Aon, E.-Y. Kim, M.A. Petr, H. Cai, C.D. Germanio, A.D. Francesco, K. Fishbein, V. Guiterrez, I.N. Enamorado, Y. Wang, J. Zhang, L. Zhang, R.G. Spencer, K.G. Becker, J.M. Egan, E.G. Lakatta, B. Gao, and R. de Cabo, Cell Metab 32:1–12, 2020; DOI:10.1016/j.cmet.2020.04.019)
NCI-CCR, NIDDK: NEW BLOOD TEST MAY HELP IMPROVE LIVER-CANCER SCREENING
CREDIT: JINPING LIU, NCI
A viral infection signature is obtained by scanning the blood for antibodies released in response to past viral infections. The signature can distinguish people who are likely to develop liver cancer from those with chronic liver disease and healthy livers.
Researchers at NIH and several academic medical centers have developed a new test that may improve the detection of liver cancer before it is symptomatic. The investigators developed a simple blood test to check for a patient’s viral infection history. Patients who have suffered from liver infections have a high risk of developing hepatocellular carcinoma (HCC), the most common form of liver cancer. Early detection of cancer is key for achieving a cure and improving survival. Emerging research suggests that past viral infections may permanently alter how the body fights diseases including cancer.
Drawing on this idea, the researchers assessed how a history of viral exposures in an individual is associated with their risk for developing HCC. They scanned the blood of 899 people, including 150 with HCC, for antibodies from more than 1,000 strains of 206 different types of human viruses. The researchers identified a viral exposure signature (pattern of antibodies)—with “footprints” from 61 different viruses—uniquely associated with HCC patients. To validate the signature, the researchers tested it on blood samples from 173 people with chronic liver disease who were part of a 20-year study. Forty-four of those patients developed HCC. The signature correctly identified those who developed HCC. It didn’t matter whether the blood samples were taken when the cancer was diagnosed or taken at the beginning of the study, up to 10 years before diagnosis. The new test could be a potentially life-saving tool for the early detection of liver cancer.
The scientists are continuing their study and plan to test the approach in clinical trials including in a prospective surveillance study of people with risk factors for HCC. (NIH authors: J. Liu, W. Tang, A. Bhudu, M. Forgues, M.O. Hernandez, J. Candia, Y. Kim, E.D. Bowman, S. Ambs, Y. Zhao, B. Tran, X. Wu, C. Koh, P. Surana, T.J. Liang, M. Rajaure, T.F. Greten, and X.W. Wang, Cell 182:1–12, 2020; DOI 10.1016/j.cell.2020.05.038)
NIDCD: DEVELOPMENTAL MAP OF INNER-EAR SOUND SENSOR IN MICE
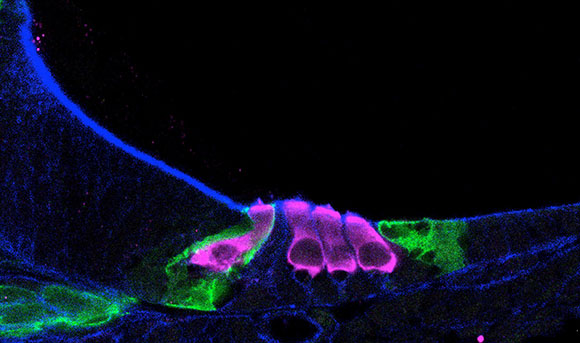
CREDIT: HELEN MAUNSELL, NIDCD
Single-cell RNA sequencing helped scientists map how sensory hair cells (pink) develop in a newborn mouse cochlea.
Much of our ability to hear is regulated by the vibrations of tiny hair cells located along the cochlea, a snail-shaped organ in our inner ear. The hair cells and the surrounding supporting cells are tightly packed together, making it difficult to separate them for individual study. NIDCD researchers and colleagues overcame this problem, however, by using single-cell RNA sequencing (RNAseq) to developed a roadmap of gene-expression patterns in developing inner-ear cells in mice. The investigators were able to separate 30,000 cochlear cells at four different developmental time points and capture individual cells for analysis with RNAseq.
Initially the researchers characterized the cells into types based on expression of known genes in inner-ear cells including inner hair cells, outer hair cells, and support cells. Then they focused on one gene: transforming growth factor beta receptor 1 (Tgfbr1), which, when mutated, has been linked to hearing loss in two connective-tissue disorders—Ehlers-Danlos syndrome and Loeys-Dietz syndrome. In mice treated with a Tgfbr1 blocker during the embryonic stage, the researchers saw fewer outer hair cells than in mice not treated with the blocker, indicating a role for Tgfbr1 in correct hair-cell development.
Understanding how these cells are formed may one day enable scientists to develop stem-cell therapeutics to treat or reverse hearing loss. The authors have made the RNAseq analysis data available on the Gene Expression Analysis Resource, a web-based platform for sharing and analyzing large datasets. (NIH authors: L. Kolla, M.C. Kelly, A. Anaya-Rocha, K. Ellis, A. Lemons, J.C. Mays, E.C. Driver, and M.W. Kelley, Nat Commun 11:article number 2389, 2020; DOI:10.1038/s41467-020-16113-y)
This page was last updated on Wednesday, March 23, 2022
