Cellular Signaling Proteins As Potential Targets for Novel Antidiabetic Drugs
NIDDK Researchers Shed New Light on the Mechanisms of Diabetes

CREDIT: YINGHONG CUI, NIDDK
Jürgen Wess’s research team recently published three papers that shed new light on the mechanisms of diabetes and show promise for possible treatments.
A research group led by Jürgen Wess in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) recently published three papers that shed new light on the mechanisms of diabetes and show promise for possible treatments. Two of the papers appeared in Science Advances and the third was published in Nature Communications.
In the first two papers, the researchers discovered two new roles that the signaling protein beta-arrestin-1 (barr1), plays in regulating important metabolic functions. Barr1 is a widely expressed cytoplasmic protein that regulates cellular responses to extracellular stimuli. The researchers used genetically modified mice to observe barr1’s effects on metabolism in brown adipose tissue (brown fat) and in certain neurons in the brain.
In the first study, Wess’s group found that barr1 is critical in maintaining the proper function of brown fat, which metabolizes nutrients to create body heat. The researchers examined metabolic functions in mice that were modified to lack barr1 and in mice that overexpressed barr1 in brown-fat cells. When fed a high-calorie diet, the barr1-deficient mice showed elevated blood-glucose concentrations and developed insulin resistance, two key features of type 2 diabetes; the mice that overexpressed barr1 maintained stable blood-glucose concentrations. (NIH authors: S.P. Pydi, S. Jain, L.F. Barella, L. Zhu, W. Sakamoto, J. Meister, L. Wang, H. Lu, Y. Cui, O. Gavrilova, and J. Wess, Sci Adv 6:eaba1733, 2020; DOI:10.1126/sciadv.aba1733)
“It clearly surprised us that beta-arrestin-1 plays such a critical role in these key metabolic processes,” said Wess.
In the second study, the NIDDK researchers demonstrated that barr1 also modulated the activity of hypothalamic agouti-related protein (AgRP) neurons, which regulate food intake and maintain proper blood-glucose concentrations. Mice that lacked barr1 in AgRP neurons displayed impaired glucose tolerance and insulin sensitivity. Mice that overexpressed barr1 in AgRP neurons were protected against obesity-associated metabolic impairments. These effects are similar to those observed with the brown fat barr1 mutant mice, but the difference lies in the mechanism—the presence of barr1 enables insulin to silence AgRP neurons when necessary. The researchers found that barr1’s overall effect is to protect metabolic function and stabilize blood glucose, which has implications for treating the harmful effects of type 2 diabetes. “This is the first study demonstrating that [barr1] is essential for maintaining whole-body glucose homeostasis and proper insulin sensitivity,” the authors wrote. (NIH authors: S.P. Pydi, Z. Cui, L.F. Barella, J. Pham, Y. Cui, O. Gavrilova, C. Buettner, and J. Wess, Sci Adv 6:eaaz1341, 2020; DOI:10.1126/sciadv.aaz1341)
In the third study, Wess’s group demonstrated that the presence of Gi-type G proteins expressed by fat cells is essential for maintaining normal blood-glucose concentrations. “Drug-mediated activation of Gi signaling in fat tissue may prove beneficial for reducing elevated blood-glucose levels in type 2 diabetes,” said Wess. (NIH authors: L. Wang, S.P. Pydi, L. Zhu, L.F. Barella, Y. Cui1, O. Gavrilova, and Jürgen Wess, Nat Commun 11:article number 2995, 2020; DOI:10.1038/s41467-020-16756-x)
“Obesity and type 2 diabetes have emerged as major threats to human health in the 21st century,” Wess said. “It is essential to develop novel antidiabetic and anti-obesity drugs that are endowed with increased efficacy and show few side effects.”
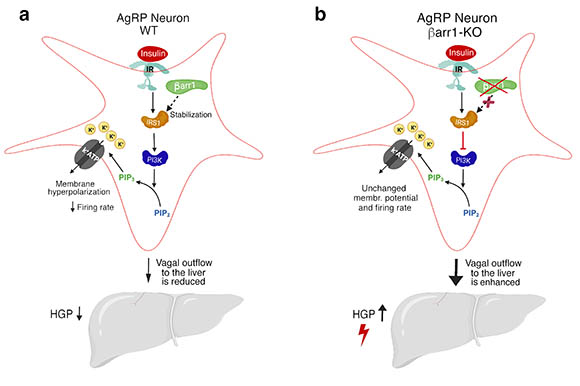
CREDIT: SAI PYDI, NIDDK
In one study NIDDK researchers demonstrated that the signaling protein, barr1, modulates the activity of hypothalamic AgRP neurons, which regulate food intake and maintain proper blood-glucose levels. Signals from these neurons help to control glucose production in the liver. Shown: a) The presence of barr1 in AgRP neurons in wild-type (WT) mice reduces the hepatic (liver) glucose production (HGP) and protects against obesity-associated metabolic impairments; b) Deletion of barr1 in AgRP neurons in knock-out mice disrupts insulin-mediated neuronal inhibition, ultimately resulting in increased HGP. Key: IR and IRS1, insulin receptors; PI3K is a kinase; PIP2 and PIP3 are membrane-bound lipid molecules; K+ = potassium; K+ATP = ATP-sensitive potassium channel; and vagal outflow represents the vagus nerve sending signals to the liver. The neuronal pathways linking the silencing of AgRP neurons to reduced vagal outflow remain to be elucidated.
This page was last updated on Wednesday, March 23, 2022
