Center for Cellular Engineering
New Name and New Location

CREDIT: DANIEL SOÑÉ
Taking part in the groundbreaking for the newest cell-processing facility are (from left) NHLBI Director Gary Gibbons; NIAID Director Anthony Fauci; NIH Deputy Director for Intramural Research Michael Gottesman; Office of Research Facilities (ORF) construction project officer Hamideh Alehossein; ORF Director Dan Wheeland; NIH Director Francis Collins; HHS Assistant Secretary for Health Admiral Brett Giroir; Clinical Center (CC) CEO James Gilman; CC Department of Transfusion Medicine Chief Harvey Klein; and CC Cell Processing Section Chief David Stroncek.
Soon you will be seeing giant construction cranes hoisting modules for a new cellular-processing facility onto the East Terrace of the NIH Clinical Center (CC), a.k.a. Building 10. The facility represents the most recent expansion of the CC’s Department of Transfusion Medicine’s (DTM’s) growing capacity to support intramural cellular-therapy protocols. On January 22, 2019, the official groundbreaking ceremony took place, with NIH director Francis Collins, hospital CEO James Gilman, Assistant Secretary for Health Admiral Brett Giroir (U.S. Department of Health and Human Services), and others on hand to celebrate. The day also marked the formal recognition of the transition of DTM’s Cell Processing Section to its new identity as the Center for Cellular Engineering (CCE).
The CCE will “enable intramural researchers to be on the leading edge” of cellular engineering, said Collins at the ceremony.
The CCE is expected to meet the increasing demand for customized cellular-therapy products and services that are needed for personalized treatments. Products include CAR-T cells for cancer immunotherapies; pluripotent stem cells to treat macular degeneration; and gene therapies for epithelial cancer. The CCE will also provide leadership, best-practices research, support, and training in cellular engineering. There are already 34 clinical-trial protocols underway that rely on the CCE; by 2020, 12 more protocols are expected to be added.

CREDIT: PERKINS+WILL, DPR CONSTRUCTION
Architectural rendering of CCE’s new modular facility to be built on the East Terrace of Building 10.
The CCE’s existing cell-processing facilities include laboratories to develop and characterize novel cellular products and 11 rooms for cellular engineering. The demand for cells is increasing rapidly so, in addition to soon-to-be-built CCE modules on the East Terrace (which are expected to be completed within a year), additional space is being renovated on the 12th floor of the CC’s E-wing. The CCE is operating under the guidance of DTM Chief Harvey Klein, Deputy Chief Bill Ward, and Section Chief David Stroncek.
Meet the Patients

CREDIT: THE CHILDREN’S INN AT NIH
During his stay at the NIH Clinical Center, seven-year-old Cole was visited by D.C. United soccer player Jalen Robinson. Under the care of Jennifer Kanakry and her team, Cole received a stem-cell transplant for a rare genetic disease.
Seven-year-old Cole is being treated at the NIH Clinical Center for a rare genetic disease called DADA2 (deficiency of the enzyme adenosine deaminase 2), caused by mutations in the ADA2 gene. The disease, which usually starts in childhood, can cause recurrent strokes, severe systemic inflammation, immune deficiency, and damage to body tissues and organs. Cole is enrolled in a clinical trial being run by Jennifer Kanakry (National Cancer Institute) who relies on the CCE to provide engineered cells.
“The trial is aimed at studying new ways to hopefully improve the safety and efficacy of [an allogeneic hematopoietic stem-cell] transplant, even for problems with the immune system where the risk of the transplant not taking [is] high,” said Kanakry. “The Center for Cellular Engineering is critical to allow us to provide a potentially life-saving, curative therapy to our patients as quickly as possible.”
NCI senior investigator Dennis Hickstein is also counting on the CCE for his research on a rare bone-marrow-failure syndrome called GATA2 deficiency. (GATA2 is a zinc-finger transcription factor.) “The [CCE] is critical for enabling freshly harvested bone-marrow cells from the donor to be infused into the recipient in a timely manner,” he said. “Bone-marrow transplantation in diseases such [as] GATA2 deficiency relies upon the ability of the normal donor cells to replace the defective bone-marrow cells. The sooner that the cells are infused, the sooner the patient will acquire a new blood and immune system.”
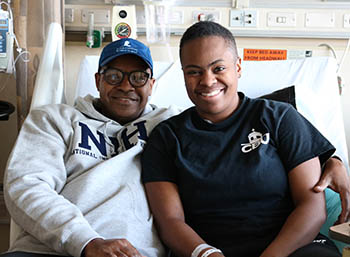
CREDIT: LIANNE PRIEDE, NCI
Jahleel, an NIH Clinical Center patient with a rare bone-marrow-failure syndrome called GATA2 deficiency, received a bone-marrow transplant of 200 million bone marrow cells per kilogram from her father (left) under the care of NCI senior investigator Dennis Hickstein and his team.
One of his patients is Jahleel, who has GATA2 deficiency. She received a bone-marrow transplant of 200 million bone-marrow cells per kilogram from her father.
To read more about Jahleel, click here.
This page was last updated on Tuesday, April 5, 2022
