Research Briefs
NINDS: NIH SCIENTISTS TRY TO CRACK THE BRAIN’S MEMORY CODES
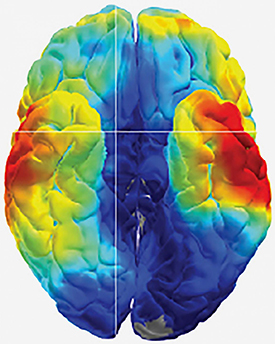
CREDIT: KAREEM AMIR ZAGHLOUL LAB, NINDS
Cracking the brain’s memory codes: Scientists at NINDS used electrical recordings to study how the human brain remembers.
In a pair of studies of patients with drug-resistant epilepsy, scientists at NINDS explored how the human brain stores and retrieves memories. One study suggests that the brain etches each memory into unique firing patterns of individual neurons. The second study suggests that the brain replays memories faster than they are stored.
To help locate the source of the patients’ seizures, the team surgically implanted a grid of electrodes into the patients’ brains and monitored electrical activity for several days. For both studies, the researchers monitored brain electrical activity while testing the patients’ memories. The patients were shown hundreds of pairs of words, such as “pencil and bishop” or “orange and navy,” and later were shown one of the words and asked to remember its mate. The results showed that the brain replays memories on fast forward. In the other study, the researchers used a new type of grid, called a high-density microelectrode array, to monitor the activity of dozens of individual neurons during the memory tests. The arrays were implanted into the middle temporal gyrus, a part of the brain thought to control word, face, and distance recognition. That study supported the idea that each memory is encoded by a unique firing pattern of individual neurons in the brain.
In the future, the researchers plan to continue exploring the neural mechanisms that underlie how the brain forms and retrieves memories and whether they can use similar techniques to understand the electrical codes underlying the epilepsies. [(1) NIH authors: R.B. Yaffe, J. Arai, S.K. Inati, and K.A. Zaghloul, J Neurosci DOI:10.1523/JNEUROSCI.3810-16.2017 (2) NIH authors: A.I. Jang, J.H. Wittig, Jr., S.K. Inati, and K.A. Zaghloul, Curr Biol 27:1700–1705e5, 2017; DOI:10.1016/j.cub.2017.05.014]
NIAID: REAL-TIME IMAGING IN MICE A PROMISING INFLUENZA STUDY TOOL

CREDIT: NIAID
The IVIS imaging system.
NIAID scientists have found a noninvasive method—real-time imaging—to track and monitor viruses, bacteria, and various types of cells and genes. The promising new method will also make it possible to evaluate whether candidate vaccines and treatments are effective in an animal model.
For the current study, NIAID scientists infected mice with a “reporter” virus after the mice had been immunized against influenza or treated with anti-influenza antibodies either before infection or three days after. The reporter virus, developed with collaborators at the University of Wisconsin at Madison, included a gene that made the virus “light up” in all infected mice. The researchers then injected the mice with a substance that would recognize the luminescent gene in an optical imaging system, allowing them to monitor infection in real time. The group used the imaging system to assess how well the experimental vaccines and antibodies protected the mice from infection and to differentiate between the interventions used, providing clues about how these prevention strategies conferred protection.
They also offered advice to researchers who are considering live imaging as an evaluation tool. For example, how the reporter virus is designed could affect its virulence, and host factors, such as inflammation, could affect detection of the luminescent signal. (NIH authors: R. Czakó, E.W. Lamirande, K.W. Bock, I.N. Moore, and K. Subbarao, mBio DOI:10.1128/mBio.00714-17, 2017)
NIAAA: STUDY FINDS TENS OF MILLIONS OF AMERICANS DRINK ALCOHOL IN DANGEROUSLY HIGH AMOUNTS
Nearly 32 million adults in the United States (13 percent of the U.S. population aged 18 and older) consumed more than twice the number of drinks considered binge drinking on at least one occasion within a year, according to a 2013 survey that asked about past-year drinking. This higher level of drinking is associated with increased health and safety risks. The study was conducted by researchers at the National Institute on Alcohol Abuse and Alcoholism (NIAAA). They analyzed data from two waves of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), which is a series of large epidemiologic surveys that examine alcohol use and its co-occurrence with drug use and related psychiatric conditions. Comparing data from the 2001–2002 and 2012–2013 NESARC waves, the researchers found that the prevalence of drinking at twofold or threefold—or even greater—the standard binge thresholds in the past year was significantly higher in the most recent NESARC wave, suggesting that more adults are engaging in extreme binge drinking now than a decade earlier. The researchers noted that their findings highlight the need to identify interventions to reduce extreme binge drinking and its negative consequences. Additional research is needed to determine how questions about peak alcohol consumption amounts can be valuable in screening for alcohol misuse as well as in assessing gender-specific risk factors and harms for drinking at extreme levels. (NIH authors: R.W. Hingson, W. Zha, and A.M. White, Am J Prev Med 52:717–727, 2017; DOI:http://dx.doi.org/10.1016/j.amepre.2017.02.014)
NCATS: REPURPOSING EXPERIMENTAL CANCER THERAPY TO TREAT MUSCULAR DYSTROPHY
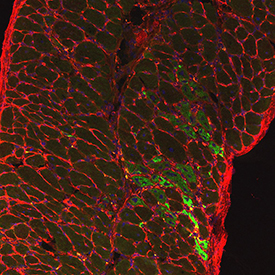
CREDIT: DEAN J. BURKIN LAB, UNIVERSITY OF NEVADA, RENO SCHOOL OF MEDICINE
Diaphragm muscle from SU9516-treated dystrophin-deficient mouse showing nuclei (blue), myofibers (outlined in red), and regenerating muscle fibers (green).
Researchers at NCATS and the University of Nevada, Reno School of Medicine (UNR Med) have demonstrated that a drug originally targeted unsuccessfully to treat cancer may have new life as a potential treatment for Duchenne muscular dystrophy (DMD). DMD is a genetic disorder characterized by progressive muscle degeneration and weakness. The candidate drug, SU9516, ramps up the muscle-repair process, helping to reinforce muscle structure. The team screened more than 350,000 compounds to find SU9516, which had been previously developed as a treatment for leukemia. The research demonstrated that this compound improved muscle function in both laboratory and animal DMD models. The results may provide a promising approach against DMD and other muscle-wasting conditions. (NIH authors: L.A. Mathews Griner, A.E. Dulcey, A. Wang, X. Xu, C.Z. Chen, X. Hu, W. Zheng, N. Southall, M. Ferrer, and J. Marugan, Mol Ther 25:1395–1407, 2017)
NIEHS: PHTHALATE EXPOSURE LINKED TO POLYDACTYLY IN MICE

CREDIT: HUMPHREY YAO, NIEHS
DEHP exposure in utero produced three additional digits in this mouse hind limb.
Phthalates, a group of compounds that soften plastic products yet also disrupt endocrine function in animals, appear in a variety of products, such as cosmetics, perfume, plastic wrap, and shampoo. An NIEHS research group found that embryos from a particular strain of mice have a higher incidence of birth defects after in utero exposure to di-(2-ethylhexyl)-phthalate (DEHP), the most widely used phthalate in the world.
The team was studying the development of sperm and testes using a genetically modified mouse strain engineered so that the male germ cells fluoresce bright green. Male and female mouse pups born to mothers given DEHP exhibited polydactyly, and the male pups produced abnormal-looking germ cells. Polydactyly (having extra fingers or toes) is the most common birth defect in humans. The team noted that phthalates have not previously been linked to severe limb malformations such as polydactyly, and more research is needed to determine whether human polydactyly is related to these findings. (NIH authors: E. Ungewitter, E. Rotgers, T. Bantukul, G.E. Kissling, and H.H. Yao, Toxicol Sci 157:8–19, 2017; DOI:10.1093/toxsci/kfx019; https://academic.oup.com/toxsci/article-lookup/doi/10.1093/toxsci/kfx019. Read the full article on the NIEHS webpage: https://www.niehs.nih.gov/news/newsletter/2017/6/papers/phthalate/index.htm.)
NHLBI, NCI: MITOCHONDRIAL “CIRCUIT BREAKER” MAY PROTECT HEART FROM DAMAGE
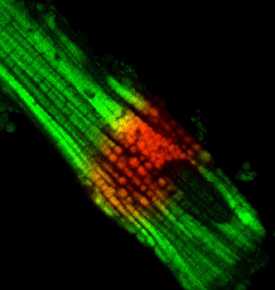
CREDIT: NHLBI
Microscopic image of mitochondria within a single heart cell. Mitochondria highlighted in red were exposed to ultraviolet light.
NIH scientists discovered biological mechanisms that appear to prevent damage to the heart muscle’s “power grid,” the network of mitochondrial circuits that provide energy to cells. One of those mechanisms, the researchers found, acts much like a circuit breaker, allowing energy to continue moving throughout the heart-muscle cells even when individual components of those cells—the mitochondria—have been damaged. In 2015, members of this same NIH research team announced the discovery of the so-called mitochondrial power grid. Since that pivotal discovery, some scientists have raised questions about how such a grid would protect itself from damage to the muscle cells. This new finding offers some key insights.
Using high-resolution 3-D images and special light-activated probes, the scientists revealed a two-part system protecting the heart muscle’s power grid from disease-related damage. Instead of being organized as one large, grid-like network such as in skeletal muscle, the mitochondrial circuits in the heart are arranged in parallel rows that form several smaller subnetworks, the researchers found. Each subnetwork acts as a mechanism to prevent damage by limiting the spread of electrical dysfunction to smaller regions. The researchers compared the newly discovered circuit-breaker mechanism to lightning striking a city power grid: Lights may flicker over the whole city, but once the circuit breaker activates, only part of the city loses power. (NIH authors: B. Glancy, L.M. Hartnell, C.A. Combs, A. Fenmou, J. Sun, E. Murphy, S. Subramaniam, and R.S. Balaban, Cell Reports 19:487–496, 2017; https://doi.org/10.1016/j.celrep.2017.03.063)
NICHD: MOLECULE IDENTIFIED THAT MAY HELP CONTROL SLEEP AND WAKE CYCLES
A molecule may work with the hormone melatonin to regulate 24-hour changes in metabolism, including sleep and wake cycles, according to researchers at the NICHD and other institutions. The researchers identified N-acetyltryptamine in the blood of humans and two animal species (rats and rhesus monkeys) and found that the molecule increases in the blood at night and decreases during the day, in parallel with concentrations of melatonin.
The researchers believe that N-acetyltryptamine is the original time-keeping compound from which melatonin later arose during the course of evolution. But because N-acetyltryptamine binds only weakly to the melatonin receptor and is present in the blood at low concentrations, it’s unlikely that it works directly with melatonin to regulate sleep and waking cycles as a hormone. It is more likely that N-acetyltryptamine works locally in tissues where it is synthesized, such as in the pineal gland.
The researchers hope to decipher the role of N-acetyltryptamine in the sleep-wake cycle. They also aim to investigate whether it also plays a role in vision. Similarly, although it is likely that N-acetyltryptamine exerts its effects through the melatonin receptor, they hope to investigate whether it interacts with other molecules, including receptors. (NIH authors: P.S. Backlund, S.L. Coon, and D.C. Klein, J Biol Rhythms 32:195–211, 2017; DOI:10.1177/0748730417700458)
NIAID, FDA: GLUTAMINE SUPPRESSES HERPES IN MICE AND GUINEA PIGS
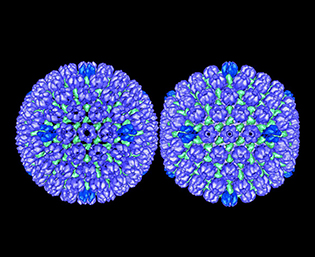
CREDIT: BERNARD HEYMANN, NIAMS
Herpes simplex virus type 1: procapsid (left) and mature capsid (right).
Glutamine supplements can suppress reactivation of herpes simplex virus (HSV) in mice and guinea pigs, according to findings recently published by NIAID and FDA researchers. There is no cure for infection with HSV-1 or HSV-2, viruses that can cause recurrent outbreaks of cold sores and genital sores in humans. Although antiviral medications can help shorten outbreaks, the virus persists in the body and can reactivate, which underscores the need for new treatment approaches.
Prior research demonstrated the importance of HSV-specific T cells for controlling recurrent HSV outbreaks, and that activated T cells require increased metabolism of glutamine (an amino acid produced by the body and found in food). Therefore, the authors speculated that glutamine supplementation might increase T-cell function and improve infection control. To test this hypothesis, scientists infected mice with HSV-1 and guinea pigs with HSV-2 and randomly assigned the animals to different treatment groups. Two weeks after infection, some animals received an oral glutamine supplement and others did not.
Results showed that mice that received glutamine were less likely to have HSV-1 reactivation than those that did not, and similarly, guinea pigs that received glutamine were less likely to have recurrent outbreaks of HSV-2 than those that did not receive the supplement. The results suggest glutamine may reduce HSV reactivation by improving the T-cell response to infection. Clinical trials are needed to determine whether this novel treatment approach would effectively treat HSV in humans, according to the authors. (NIH authors: K. Wang, Y. Hoshino, K. Dowdell, T.G. Myers, M. Sarmiento, L. Pesnicak, and J.I. Cohen; FDA authors: M. Bosch-Marce and P.R. Krause, J Clin Invest 127:2626–2630, 2017; DOI:10.1172/JCI88990; https://www.jci.org/articles/view/88990)
NIEHS: AN ANTIDEPRESSANT MAY ENHANCE DRUG DELIVERY TO THE BRAIN BY INHIBITING THE BLOOD–BRAIN BARRIER
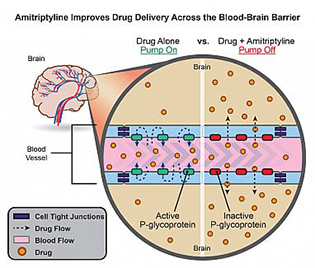
CREDIT: NIEHS
Usually, permeability glycoprotein (P-glycoprotein) prevents most medicines from entering the brain by pumping them back into the bloodstream (left). The addition of amitriptyline temporarily turns off P-glycoprotein pumps, allowing drug molecules to cross the blood–brain barrier (right).
NIEHS researchers found that, in rats, pairing the antidepressant amitriptyline with drugs designed to treat central nervous system diseases enhances drug delivery to the brain by inhibiting the blood–brain barrier. Although researchers caution that more studies are needed to determine whether people will benefit from the discovery, the new finding has the potential to revolutionize treatment for a whole host of brain-centered conditions, including epilepsy, stroke, human amyotrophic lateral sclerosis, and depression. The results are so promising that a provisional patent application has been filed for methods of co-administration of amitriptyline with central nervous system drugs. (NIH authors: D.B. Banks, G.N.Y. Chan, R.A. Evans, D.S. Miller, and R.E. Cannon, J Cereb Blood Flow Metab DOI:10.1177/0271678X17705786; https://www.ncbi.nlm.nih.gov/pubmed/28447863)
NICHD: KEY REGULATOR OF FETAL GROWTH IN MICE
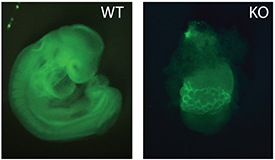
CREDIT: P. YANG, T. MACFARLAN, NICHD
Microscopy image of a normal mouse embryo (left) and one that is missing ZFP568 (right).
A protein called zinc finger protein 568 (ZFP568) regulates an important fetal growth hormone called insulin-like growth factor 2 (IGF-2), according to a mouse study led by researchers at NICHD. The study is one of the first to show that Krüppel-associated box-ZFPs (KRAB-ZFPs), which are well-known for silencing viral genes left over from ancient infections, can also play an essential role in fetal and placental development.
The team discovered that ZFP568 prevented a placental version of IGF-2 from being expressed too early, during a window of time soon after the embryo implants in the uterus. Precise expression of IGF-2 is important because small changes in IGF-2 concentrations can lead to under- and overgrowth conditions in people. In the study, fetal mice lacking ZFP568 failed to develop normally, suggesting that too much IGF-2 is toxic in early development. The researchers also found that mammals, including humans, have ZPF568-like proteins, indicating that its IGF-2 suppression may have played an important role in the early evolution of mammals. The study team is exploring whether ZFP568 has a similar function in humans and whether other KRAB-ZFPs have evolved to aid in other essential developmental processes. (NIH authors: P. Yang, Y. Wang, D. Hoang, M. Tinkham, M.A. Sun, G. Wolf, M. Baker, and T.S. Macfarlan, Science 356:757–759, 2017; DOI:10.1126/science.aah6895; https://www.ncbi.nlm.nih.gov/pubmed/28522536)
NIAID: IMMUNE RESPONSES DRIVING OBESITY-INDUCED LIVER DISEASE
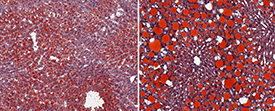
CREDIT: NIAID
The image on the left shows steatosis (fat build-up, red) in the liver of a normal mouse fed a high-fat diet. The right image shows markedly higher steatosis in the liver of a mouse lacking IFN-gamma fed a high-fat diet.
New findings from mouse models reveal that the type of immune response that helps maintain healthy metabolism in fatty tissues, called type-2 immunity, also drives obesity-induced nonalcoholic fatty liver disease (NAFLD). The work, led by NIAID scientists, shows that the inflammatory environment in the fatty liver is more complex than previously thought. These insights may inform the development of new NAFLD treatments as well as immune-altering therapies for obesity and related health issues in people with NAFLD. Although the mechanisms regulating NAFLD progression have been unclear, scientists had suspected that the type of immune response that inflames fatty tissues during obesity, known as type-1 immunity, also worsens liver disease.
Unexpectedly, the NIAID-led team found that obese mice engineered to have strong type-1 immune responses were resistant to nonalcoholic steatohepatitis (NASH), liver inflammation and damage caused by the buildup of fat in the liver. This protection was associated with activation of a type-1 immune molecule called interferon-gamma (IFN-gamma). Obese mice lacking IFN-gamma progressed rapidly to NASH and developed liver scarring.
Further experiments revealed that NASH-induced scarring depends on transforming growth factor–beta (TGF-beta), a broad immune system regulator and target for experimental NASH therapies, and interleukin-13 (IL-13), a type-2 molecule produced by various immune cells. Blocking TGF-beta in mouse models slowed NASH-driven liver scarring, but the number of eosinophils and amount of IL-13 produced in the liver increased to compensate for the lost TGF-beta function. Simultaneously blocking TGF-beta and IL-13 more effectively stopped liver scarring.
To determine whether these findings may be relevant to human disease, the scientists examined liver biopsies from people with NASH. As they saw in mice, the researchers observed evidence of type-2 inflammation and accumulation of eosinophils. The researchers next plan to use mouse models to assess the specific roles of eosinophils in the liver and to study ways to specifically block the IL-13 and TGF-beta cell-signaling that leads to liver scarring. (NIH authors: K.M. Hart, J.C. Sciurba, R.L. Gieseck, K.M. Vannella, T.H. Acciani, R. de Queiroz Prado, R.W. Thompson, S. White, T.R. Ramalingam, and T.A. Wynn, Sci Transl Med 9:eaal3694, 2017; DOI:10.1126/scitranslmed.aal3694; http://stm.sciencemag.org/content/9/396/eaal3694.full)
NICHD: CELL PARTICLES MAY HELP SPREAD HIV INFECTION
CREDIT: NIAID
HIV-infected immune cell.
The human immunodeficiency virus (HIV) appears to enlist the aid of nano-sized structures released by infected cells to infect new cells, according to a study by NICHD researchers. The scientists discovered that cells infected with HIV appear to produce extracellular vesicles (EVs) that manipulate prospective host cells to pass infection to other cells.
In the current study, the researchers isolated HIV and EVs from infected cultures, separated the two, and then tested the ability of HIV to infect new cultures—both in the presence of EVs and on its own. Because EVs are extremely tiny and difficult to sort with conventional techniques, the researchers devised a new technology to isolate and study them.
They chemically attached magnetic nanoparticles to antibodies. After the magnetic nanoparticle-carrying antibodies latched onto its target molecule on the surface of an EV or virus, the researchers used magnetic forces to pull the viruses or EVs out of solution. They found that EVs produced by HIV-infected cells also carry an HIV protein on their surfaces, envelope glycoprotein 120 (gp120), which HIV uses to bind to cells in the infection process.
When the researchers added the EV-depleted sample to blocks of human lymphatic tissue, HIV infections in the cultures were 55 percent lower, compared to a control sample that retained EVs. The researchers believe that the loss of the gp120 that would have been provided by the EVs (had they not been removed from the HIV cultures) was responsible for the drop in HIV infection.
Although EVs lack the HIV RNA to infect a cell, the researchers theorize that the gp120 on the EV surface can interact with the host cell, allowing HIV to infect it more easily. (NIH authors: A. Arakelyan, W. Fitzgerald, S. Zicari, C. Vanpouille, and L. Margolis, Sci Rep, DOI:10.1038/s41598-017-01739-8)
This page was last updated on Saturday, January 18, 2025
