The Thymus: A Small Organ with Huge Immunological Impact
Special from the 2016 NCI Thymus Symposium
Scientists once thought that the thymus—a little organ in your upper chest—was a vestigial leftover without a major function. It wasn’t until the early 1960s that Australian immunologist Jacques Miller made an amazing discovery: The thymus is the place where T cells, the major effector cells of the immune system, are generated.
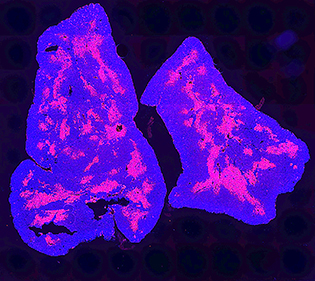
CREDIT: TEJAS KADAKIA, NCI
This image of a mouse thymus shows all cells in the thymus (blue); the red portion indicates the thymus medulla.
We now know that the thymus receives percursors of T cells, called thymocytes, from the bone marrow and helps them to mature into full-fledged T cells (such as CD4 or CD8 cells) that are trained to attack foreign cells. NIH researchers have played a key role in moving the field of thymus research forward.
Transforming the field
In 2000, Alfred Singer’s group at the National Cancer Institute (NCI) transformed the field of thymus biology by demonstrating a novel selection mechanism for thymocyte lineage choice and maturation, called the “kinetic signaling” model (Immunity 13:59–71, 2000). A major revelation of this study was that lineage fate decisions are made by the “kinetics,” and not the “strength,” of the T-cell receptor signaling as previously thought. This seminal finding was highlighted in 2016 in the Journal of Immunology as a “Pillar of Immunology” article for its countless citations, for changing the conventional view on thymocyte selection and maturation, and for advancing thymus research (J Immunol 196:1985–1997, 2016).
In 2012, Singer with Remy Bosselut (NCI) instituted the inaugural NIH Thymus Symposium to highlight thymus-focused immunology basic research. Since then, there has been an explosion of new discoveries and information so that the next thymus symposium was certainly overdue. After four years, in December 2016, the second NIH Thymus Symposium was held to gather new insights on thymus research, examine the implications, and spark collaboration among thymus-research enthusiasts. Attendees and presenters included scientists and fellows from within the NIH and as far away as France and Japan.
New Insights and implications
Thymocytes, the precursor of T cells, develop in a region of the thymus called the thymic cortex; selected thymocytes proceed to the thymic medulla and become mature T cells; and eventually the T cells are released into the bloodstream. Yousuke Takahama (Tokushima University in Tokushima, Japan) described how the protein C-C motif chemokine receptor type 7 (CCR7) is essential for the cortex-to-medulla migration and for establishing self-tolerance, thereby preventing tissue-specific autoimmunity. Consequently, he found that in mice, the deletion of CCR7’s binding partners–C-C motif ligands 21A and 19—had a negative effect on establishing immune tolerance.
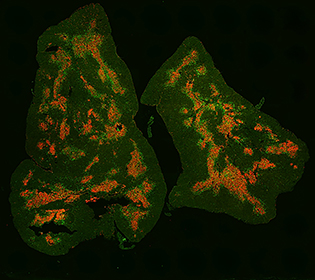
CREDIT: TEJAS KADAKIA, NCI
This image of a mouse thymus shows regulatory T cells (green) and the thymus medulla (red).
Wanjun Chen (National Institute of Dental and Craniofacial Research) elucidated the essential role that transforming growth factor–beta plays in the development of immune-suppressive regulatory T cells (Tregs). Understanding how Tregs develop might lead to the identification of molecular-targeted therapies for autoimmune diseases (Proc Natl Acad Sci U S A 111:E465–473, 2014).
Although it was once thought that the maturation of T cells took place entirely within the thymus, recent evidence has shown otherwise. The current editor-in-chief of the Journal of Immunology, Pamela Fink (University of Washington in Seattle), provided clues about how newly generated T cells, called recent thymic emigrants (RTEs), go through a final maturation step outside the thymus. Before they mature, RTEs are ineffective at mounting adequate immune responses. Understanding RTE biology is important because they are overrepresented in neonates and in adults recovering from lymphophenia, and may play a role in several diseases including ulcerative colitis, chronic myeloid leukemia, and autoimmune thyroid disease.
After listening to several other enlightening talks on thymus-related research, the scientists were eager to interact with the presenters and each other at the reception at the end of the day. Some of the interactions may stimulate collaborations that will push the field of thymus research even further by the time the next symposium comes around.
More Glimpses into the Thymus
Naomi Taylor (Institute of Molecular Genetics, Montpellier, France)
“Intrathymic Gene and Stem-cell Targeting for T-cell Reconstitution: A Glimpse into the Thymus”
Naomi Taylor studies T-cell differentiation in genetic and acquired immune deficiencies and cancers in humans and mice. At the symposium, she spoke about intrathymic gene and stem-cell targeting as a therapeutic approach for immunodeficiency. Conventional cell therapy for immunodeficiency—using donor bone-marrow progenitor cells—often fails because of the lack of histocompatible sibling donors or because the progenitors seeded in the thymus are unable to sustain their viability. Taylor’s lab studies gene-therapy approaches to manipulate defective thymocytes. Her lab uses adeno-associated viral vectors (AAV) in ZAP70-deficient mice, which lack CD8-positive T cells. After AAV-mediated gene therapy of the ZAP70 gene was administered directly to the thymus, the T-cell count improved and the percentage of both CD4 and CD8 cells increased. Preclinical studies are also being performed on nonhuman primates. Indeed, intrathymic gene therapy shows promise as a way to treat immunodeficiency.
Paul Love (Eunice Kennedy Shriver National Institute of Child Health and Human Development)
“A Mechanism of THEMIS Function”
Paul Love’s lab focuses on mammalian hematopoiesis and has a long history of studying the process of T-cell development, which begins when multipotent progenitor cells, derived from hematopoietic stem cells in the bone marrow, migrate to the thymus. In 2009, his lab identified thymocyte-expressed molecule (THEMIS), a signaling protein that is critical in lymphocyte maturation and controls the fate of lymphocytes (Nat Immun 10:840–847, 2009) [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2848698/]. Love is particularly interested in investigating signal-transduction molecules and pathways that regulate T-cell maturation in the thymus. THEMIS—named for the Greek Titaness who advised the chief god, Zeus, on the rules of fate—is involved in lymphocyte maturation and controls the fate of lymphocytes as they differentiate. The Love lab is continuing to study the intricate details of signaling pathways in lymphocyte maturation and the role of THEMIS.
The 2016 NCI Thymus Symposium, held on December 9, 2016, in NIH’s Porter Neuroscience Research Center (Building 35), was chaired by Dinah Singer (NCI), Paul Roche (NCI), Vanja Lazarevic (NCI), and Richard Hodes (National Institute on Aging) and sponsored by the NCI Center of Excellence in Immunology and the NIH Cytokine Interest Group.
This page was last updated on Monday, April 11, 2022
