Ambition, and Lots of Fruit Flies
Mihaela “Ela” Serpe Explores Cell Signaling
Mihaela “Ela” Serpe credits Nobel laureate George Emil Palade for inspiring her in the 1990s when she was a research associate at the Institute of Cellular Biology and Pathology (Bucharest, Romania). Palade, who had discovered the ribosome, shared the 1974 Nobel Prize for Physiology or Medicine with two others “for their discoveries concerning the structural and functional organization of the cell.”

Mihaela “Ela” Serpe
Serpe attended a seminar Palade gave at the institute and was impressed by his insistence that although the discovery of cell structures was important, what really deserved recognition was the discovery that phosphorylation (the addition of phosphate groups to molecules) is critical for many cellular processes. It changes the activity and subcellular distribution of proteins, an entry point to understanding how cells talk to each other.

CREDIT: HUU THO NGUYEN, NICHD
Ela Serpe (back row, center) and members of her lab are enjoying a light moment posing in front of the Santiago Ramón y Cajal exhibit in the Porter Neuroscience Building. Pictured, from left: (back row) Peter Nguyen, Lindsey Friend, Serpe, Qi Wang, Shelby Newsad; (front) Rosario Vicidomini, Tae Hee Han.
And that’s what Serpe has been trying to figure out ever since. Now a senior investigator at the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), she is trying to elucidate molecular mechanisms that regulate cell-to-cell communication. In particular, she is studying how synapses form and optimize their function via extracellular signals. Those molecular messengers accumulate at high concentrations at the synapse but are only deployed at very specific times and places.
This specificity means that “all the extracellular communication is guarded” very carefully, Serpe said. She has devoted her career to investigating how all those signaling molecules get organized and concentrated at specialized cell-cell interaction zones, including glutamatergic synapses. She uses Drosophila melanogaster neuromuscular junctions (NMJ) as a model because the glutamatergic synapses of the fruit fly and human are highly similar. And fruit flies are more readily manipulated genetically so scientists can identify and characterize key components of the nervous system.
Discovery
After coming to NIH in 2008, Serpe discovered something big: a novel modulator of trans-synaptic signaling called Neto, which stands for Neuropilin and Tolloid-like—critically involved in synaptic development. The significance of the discovery was not immediately apparent because fly embryos that lack the neto gene initially develop just fine due to the maternal supply of mRNAs and proteins to the egg. It’s only after the supply of the Neto protein runs out at the end of embryogenesis that the synapses fail to form and paralysis sets in. Serpe and colleagues demonstrated that Neto is required for the initial clustering and normal functioning of glutamate receptors on the postsynaptic side of the NMJ.
Beginnings
Serpe studied how yeast cells sense and respond to their environment as she was pursuing a Ph.D. in biochemistry at the State University of New York at Buffalo (Buffalo, New York). Her research led to an invited seminar at a Gordon Research Conference on microbial stress response (Henniker, New Hampshire), after which she was offered several postdoctoral positions. She did her postdoctoral training in developmental biology and neurobiology at the University of Minnesota and the Howard Hughes Medical Institute (Minneapolis), where she entered the world of intercellular signaling and development. Since then her natural inclination to apply analytical thinking to neuroscience has suited her well.
After completing her postdoctoral training, Serpe arrived at NICHD in 2008. “You are here to do cutting-edge science that would not be funded in the extramural world,” she was told. “[A]nd you are expected to be as productive at the bench as two good postdoctoral researchers,” presumably because she wouldn’t have the grant-writing or teaching responsibilities that most extramural researchers do.
She has built her lab from the simple beginning of two summer students, who generated the first neto-null mutants, to her current full-fledged lab comprising a mix of electrophysiologists, geneticists, molecular and cell biologists, and imaging experts. The breadth of research strategies she uses to answer scientific questions is a product of her broad background and hard-won collaborations.
A favorite collaborator, Mark Mayer (NICHD), was skeptical when Serpe suggested that the ionotropic glutamate receptors she was studying were heterotetramers (four subunits, all different) that required Neto for their function. But it turned out her hypothesis was correct. And Serpe persuaded electrophysiologist Bing Zhang from the University of Missouri (Columbia, Missouri) to come to NIH to help set up an electrophysiology rig and guide a postbac on how to use it. Convincing others to join a scientific mission is a notable quality, and Serpe prides herself on forging many fruitful collaborations.
Mentorship
Mentorship is a common theme that runs through Serpe’s experience. “Nobody clipped my wings,” she said. “I was never limited in what I was trying to learn.” So she isn’t about to limit her trainees.
One of Serpe’s early mentors at NIH—the late Howard Nash (National Institute of Mental Health)—reinforced that philosophy. He encouraged her to help trainees pick their own projects, which gives them ownership.
Serpe personally trains all the researchers in her lab, ensuring that her high standards are met and that she stays engaged in the fun of doing research. In fact, it was when she was teaching her trainees how to inject dye into fly embryos that she first discovered that the neto-null mutants were powerful models for synapse development and NMJ functionality. The control embryos hatched and squirmed away from the dye needles but the mutants lacking the neto gene were completely paralyzed. Her hands-on approach to training yields better relationships and brings an extra pair of eyes to the data as they unfold.
Serpe enjoys mentoring budding scientists. “My people are growing nicely,” she said. “I’m training them and getting them to be the best that they can be.”
Dedication, collaboration, and mentorship are three qualities that make for an excellent scientist. Serpe brings all three to her lab every day.
On January 27, 2017, Mihaela Serpe gave a Director’s Seminar Series lecture and described her work on the molecular mechanisms of synapse assembly and maturation in Drosophila melanogaster. To watch a videocast of her talk go to https://videocast.nih.gov/launch.asp?21101.
A Stunning Observation
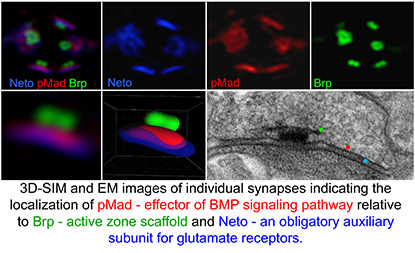
At the NIH, I focused on a novel modulator of a particular class of extracellular signals, the bone morphogenetic proteins (BMPs). I soon realized that this molecule, called Neto (Neuropilin and Tolloid-like), was essential for synapse development: In the absence of Neto the NMJ synapses fail to form and the fly embryos die completely paralyzed. My colleagues and I demonstrated that Neto is an obligatory subunit of glutamate-receptor complexes required for the clustering of receptors and the formation of functional NMJs. When my group examined the BMP signaling in mutants with disrupted Neto (or glutamate receptor) activities, we made a stunning observation: The effector of BMP pathway, a transcription factor, accumulates at synaptic terminals in response to synapse activity. We had discovered a completely new, genetically distinct, BMP-signaling modality that senses synapse activity and, in turn, mediates the recruitment of selective glutamate receptors.
As I described my group’s findings at various talks and meetings, I learned that other laboratories have observed similar BMP-signaling complexes in mice and frogs, but these complexes were never linked before to synapse function and modulation.
Selected Publications
- Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev 2012;26(9):974-987
- Peluso CE, Umulis D, Kim YJ, O'Connor MB, Serpe M. Shaping BMP morphogen gradients through enzyme-substrate interactions. Dev Cell 2011;21(2):375-383
- Han TH, Dharkar P, Mayer ML, Serpe M. Functional reconstitution of Drosophila melanogaster NMJ glutamate receptors. Proc Natl Acad Sci U S A, 2015;112(19):6182-6187
- Sulkowski M, Kim YJ, Serpe M. Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Development, 2014;141(2):436-447
- Ramos CI, Igiesuorobo O, Wang Q, Serpe M. Neto-mediated intracellular interactions shape postsynaptic composition at the Drosophila neuromuscular junction. PLoS Genet, 2015;11(4):e1005191
This page was last updated on Monday, April 11, 2022
