Research Briefs
NIDA: RESETTING THE ADDICTED BRAIN
Could drug addiction treatment of the future be as simple as an on-off switch in the brain? A study in rats has found that stimulating a key part of the brain reduces compulsive cocaine-seeking behavior and suggests the possibility of changing addictive behavior generally. NIDA researchers used an animal model of cocaine addiction in which some rats exhibited addictive behavior by pushing levers to get cocaine even when followed by a mild electric shock to the foot. Other rats did not exhibit addictive responses.
The NIDA scientists compared nerve-cell firing patterns in both groups of rats by examining cells from the prefrontal cortex. They determined that cocaine produced greater functional brain deficits in the addicted rats. The researchers then used optogenetic techniques on both groups of rats—essentially, shining a light onto modified cells to increase or lessen activity in that part of the brain. In the addicted rats, activating the brain cells (thereby removing the deficits) reduced cocaine-seeking behavior. In the nonaddicted rats, deactivating the brain cells (thereby creating the deficits) increased compulsive cocaine seeking.
This is the first study to show a cause-and-effect relationship between cocaine-induced brain deficits in the prefrontal cortex and compulsive cocaine seeking. The results provide evidence for a cocaine-induced deficit within a brain region that is involved in disorders characterized by poor impulse control, including addiction. The researchers hope that the findings can lead to treatments that would reduce compulsive cocaine seeking and craving in patients. (NIH authors: B.T. Chen, H.-J. Yau, C. Hatch, I. Kusumoto-Yoshida, A. Bonci; Nature 496:359–362, 2013)
NICHD, NINDS, NCI: NEW SYNDROME LINKED TO A SOMATIC HIF2A MUTATION
A team of NIH researchers, in collaboration with scientists from the University of Utah (Salt Lake City) and Tufts Medical Center (Boston), have identified a new syndrome involving two rare neuroendocrine tumors and a rare blood disease. The syndrome was observed in four female patients who had multiple paraganglioma and somatostatinoma tumors and the blood disease polycythemia.
Somatic mutations in the gene that encodes hypoxia-inducible factor–2-alpha (HIF2A) cause increased production of erythropoietin, which leads to increased red blood cell production called polycythemia. The mutations increase HIF2A stability and enhance its functional capacity by extending its half-life.
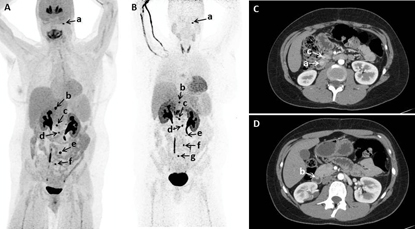
Courtesy of K. Pacak, NICHD
Functional and anatomic imaging; (A, B) 18F-fluorodopa positron emission tomography (PET)/computed tomography (CT) showing multiple tumors in a patient; (C, D) Early arterial phase of axial CT of abdomen performed with negative enteric contrast showing small masses in another patient.
Symptoms of the new syndrome include high blood pressure, heart palpitations, headaches, and anxiety. Polycythemia in the four women was found either at birth or in early childhood. All developed tumors later in life (the paragangliomas were in the abdomen and somatostatinomas in the duodenum). It is not clear whether the syndrome also exists in men.
Increasing HIF expression has been shown in many tumors, but HIF mutations have never been reported in tumors. The research is the first to provide direct evidence of HIF involvement in tumorigenesis and suggests that inhibiting HIF2A may be a way to treat the disease. The team is currently exploring that avenue. (NICHD authors: K. Pacak, I. Jochmanova, T. Prodanov; NCI authors: M. Merino, T. Fojo; NINDS authors: Z. Zhuang, C. Yang; J Clin Oncol 31:1690–1698 2013)
NIEHS: DISCOVERY OF GENE THAT IS MUTATED IN NEURODEGENERATIVE DISEASE
An international research team that included several NIEHS scientists has identified a novel factor that removes poly[adenosine diphosphate (ADP)–ribose] chains from proteins. Originally called C6orf130, the scientists renamed this gene TARG1 and its protein terminal ADP-ribose protein glycohydrolase (TARG1) because it cuts off ADP-ribose and poly(ADP-ribose) units that are directly attached to proteins. Individuals who inherit two defective copies of the TARG1 gene suffer from a progressive neurodegenerative disease characterized by seizures, lack of tendon reflex, and a weakened swallowing reflex.
The tagging of ADP-ribose chains to proteins controls gene expression, cell death, and cellular responses to DNA damage. Using X-ray crystallography and cell biological and biochemical approaches, the research team demonstrated that TARG1, a member of a large class of macrodomain proteins, homes in on and erases ADP-ribose tags. The work shows that this TARG1 action is critical for cells to coordinate normal DNA repair processes in support of proper cellular function. Future work is required to gain a better understanding of when and where TARG1 acts to regulate cellular functions. (NIEHS authors: C.D. Appel, M.J. Schellenberg, J.G. Williams, J. Krahn, R.S. Williams; EMBO J 32:1225–1237, 2013)
NIDDK: ACTIVITATING THE BETA CELL PATHWAY TO PROTECT AGAINST DIABETES
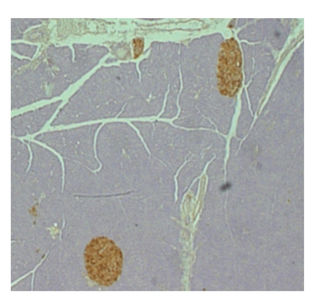
SHALINIL JAIN, NIDDK
Activation of a novel signaling pathway in beta cells maintains healthy mouse pancreatic islets (brown).
In type 2 diabetes (T2D), pancreatic beta cells fail to release enough insulin to maintain blood glucose concentrations within a normal range. So scientists are trying to develop therapeutic strategies that would improve the function of defective pancreatic beta cells.
NIDDK researchers recently demonstrated that stimulating a specific type of cell-surface receptor activated a novel beta cell–signaling pathway that had multiple metabolic benefits in mice. The mice were protected against experimentally induced diabetes and glucose intolerance that had been provoked by either streptozotocin (a toxin that selectively destroys beta cells) or an energy-rich, high-fat diet. The findings provide a rational basis for the development of anti-diabetic drugs targeting this class of receptors. (NIDDK authors: S. Jain, I. Ruiz de Azua, H. Lu, J.-M. Guettier, J. Wess; J Clin Invest 123:1750-1762, 2013)
This page was last updated on Thursday, April 28, 2022
