Recapturing Kids’ Bone-Healing Magic
IRP Study Suggests Immune System Changes Hinder Healing in Adults

IRP researchers are trying to identify why children and teens are much better at fixing bone injuries than adults, with the goal of leveraging their regenerative abilities for older individuals.
With all the hijinks that young children and teenagers get up to, it’s a good thing they heal so quickly from a broken leg or arm. Unfortunately, this ability begins to diminish within a few years of graduating high school. Now, new IRP research is showing that changes in the immune system underlie the difficulties older individuals have with repairing their bones.1
Many people have experienced a bone fracture — a partial or complete break in a bone — at some point in their lives. Fortunately, fractures eventually heal in people of any age with proper treatment, albeit more slowly in adults than in young people. However, if someone of any age ends up with a missing piece of bone much bigger than a centimeter due to a more serious trauma, that gap will never naturally fix itself. This is a particular concern when that missing piece of bone is in an area that does a lot of work, like the jaw or a leg.
“If you have a gap in your jaw bone, you can’t chew,” explains IRP investigator Janice Lee, D.D.S., M.D., the new study’s senior author. “And you can’t insert a metal plate there because the plate can’t withstand the forces of chewing forever. A plate will eventually break and needs to be replaced with a bone graft from another part of the body. These procedures add more risk to the individual and require another surgery site besides the site of injury. Even in long bones like the leg, if a large piece of bone is missing, you can’t put a plate across that gap and expect a person to be able to walk on that forever.”
Anybody can fix a fracture like this one given proper medical care and enough time, but children and teens have a unique ability to bounce back from certain forms of more extensive bone damage.
There is an important exception to this rule, however: the ribs, which can fully repair larger missing sections in young people who have not finished growing. This comes in handy because the ribs can be a source of bone for bone grafts in younger patients, since bone taken from their ribs and transplanted to another bone will eventually regenerate. Unfortunately, the same can’t be said for older individuals, since their ribs lack the ability to regenerate. In the new study, Dr. Lee and the paper’s first author, IRP postdoctoral fellow Luciana Yamamoto de Almeida, D.D.S., Ph.D., aimed to figure out why.
To do so, they began by removing a bit of bone from the ribs of mice younger than two months of age or older than ten months. Just like in humans, the missing piece of rib regenerated in the younger mice but not the older mice, which filled the gap in the bone with scar tissue and fat instead. Part of the reason for this difference was that, in the younger mice, genes involved in the production of bone and cartilage — a crucial secondary component of strong bones — were more active in the damaged area of bone compared to in the older mice.
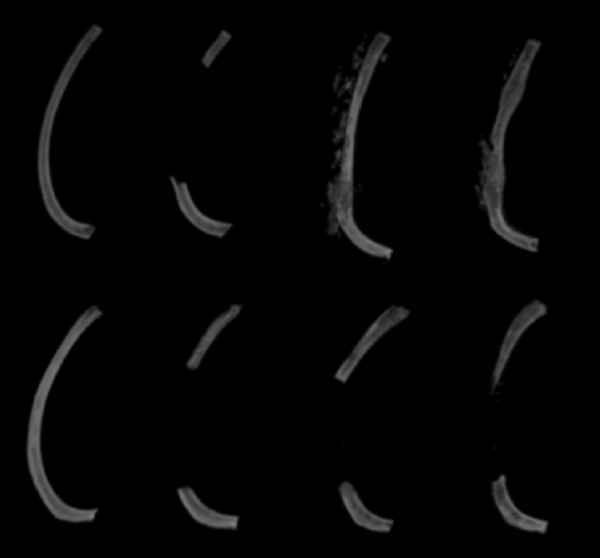
As shown in this representative image from the study, immature mice (top row) regenerated their missing section of rib bone over time while mature mice (bottom row) did not, even 60 days after the section was removed (far-right column).
“These genes are being suppressed in the mature mice,” says Dr. Yamamoto de Almeida.
“But what is suppressing them — that’s where the immune cells have their role,” adds Dr. Lee. “As you get older, these immune cells start to alter the ability of the bone- and cartilage-producing cells to do their job.”
Indeed, two types of immune cells — B cells and macrophages — showed up in much larger numbers at the area of damaged bone in younger mice than in older mice. What’s more, four immune system molecules were more abundant at the injury site in younger mice than in older mice, including a substance important for the movement and function of macrophages, another involved in the maturation of B cells, and one that helps draw both of those cell types to the site of an injury.

Dr. Janice Lee (left), the study’s senior author, and Dr. Luciana Yamamoto de Almeida (right), the study’s lead author
To further explore the involvement of the immune system in rib regeneration, the researchers examined rib repair in young mice missing various genes involved in the immune system. Although their ribs did ultimately heal completely, mice missing genes involved in the function of B cells and macrophages actually produced too much bone, with altered architecture, early in the regeneration process. What’s more, mice lacking a gene called CCR2 that helps bring macrophages to the site of an injury produced significantly less collagen, which is crucial for cartilage formation, compared to genetically typical mice and had lower bone density three weeks into the repair process.
“This suggests that the bone will be lower-quality and more fragile,” Dr. Yamamoto de Almeida says.
Finally, the researchers attempted to bolster rib regeneration in older mice by connecting their blood circulation to that of younger mice through a process known as ‘parabiosis.’ Much to their consternation, even though past studies had shown parabiosis between one non-injured immature mouse and one injured mature mouse helps bone fractures in the older mouse heal, it did not help older mice regenerate large gaps in their ribs. Only when a piece of bone was removed from a rib in both the older mouse and the younger mouse did parabiosis lead to robust rib regeneration in the older mouse, suggesting that the injury in the younger mouse caused its body to produce some sort of bone-healing molecular cocktail that spread to the older mouse via the animals’ shared blood supply.

Fractures caused by falls are among the most common injuries experienced by older adults. The new study’s results will help further the search for ways to help them recover from these injuries and possibly even prevent them by preventing age-related weakening of their bones.
Discovering exactly which substances produced by the young mice spurred bone regeneration in the older mice will be a key next step for Dr. Lee’s lab, as well as determining the timing and quantity of their release. If her research group can answer those questions, they might be able to come up with a way to help humans of all ages recover from bone injuries, and even potentially prevent the weakening of bones that occurs in millions of Americans aged 50 and older.
“If we can figure out what to give adults that they need and are missing, it may allow them to repair these bone injuries, and maybe someday we can also help them with osteoporosis, which is not damage but something about the aging body where it no longer wants to form bone,” Dr. Lee says. “The bone regeneration slows down for many unknown reasons. We hope to reverse some of this age-related inability to make bone.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] de Almeida LY, Dietrich C, Hanner AS, McTighe KM, Martin D; NIDCD/NIDCR Genomics and Computational Biology Core; Fairbanks T, Link TM, Le JM, Curry N, Jani P, Gao X, Yu W, Mariani FV, Duverger O, Lee JS. Skeletal Maturity and Age-Related Changes in Immune Cells and Circulatory Factors Impair Large-Scale Bone Regeneration. Aging Cell. 2025 Jul 21:e70177. doi: 10.1111/acel.70177.
Related Blog Posts
This page was last updated on Monday, September 8, 2025
