Preventing Cellular Rust Hinders Tuberculosis
Study Suggests New Treatment Approach for Deadly Lung Infection
The bacteria responsible for tuberculosis, pictured here, cause more than a million deaths each year. New IRP research could help improve treatment for the illness.
Oxygen is, quite literally, the air we breathe (or, more accurately, 21 percent of it). However, just as oxygen in the air can turn a handy garden tool into a useless hunk of rust, certain unstable, oxygen-containing molecules in our bodies can wreak havoc on our cells. According to new IRP research, revving up cellular systems that prevent this kind of damage could significantly improve outcomes for people with tuberculosis.1
A bacterial infection that initially begins in the lungs, tuberculosis remains a leading cause of death globally. In fact, in recent years, the only infectious disease with a greater death toll was COVID-19. Most tuberculosis deaths occur in developing countries, where public health infrastructure and access to medical care are poorer than in nations like the U.S. Despite the effectiveness of antibiotics against the illness, those systemic issues can make treatment tricky in the places where tuberculosis is most common.
“Antibiotics are really effective, but the problem with antibiotic therapy is you need a long-term treatment to make sure you kill most of the bacteria,” explains IRP research fellow Eduardo Amaral, Ph.D., the study’s first author. “This is quite complicated because after a couple months of the therapy, the patient starts to feel better, and often stops the treatment due to several reasons, including the antibiotics’ side effects.”
“That’s why we’re trying to find an alternative approach to combine with current antibiotic therapies to try to shorten the treatment regimen,” he continues. “Instead of needing to be on antibiotics for six months to a year, they could just be on them for three or four months.”
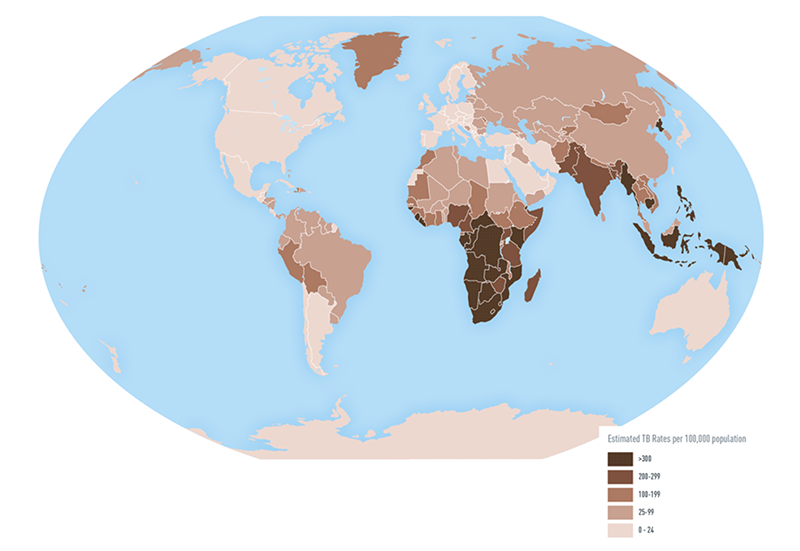
This map from the U.S. Centers for Disease Control and Prevent (CDC) shows the disparate rates of tuberculosis around the world. The map is based on data from 2016.
While that may seem like a small change, enabling antibiotics to wipe out tuberculosis more quickly would be a boon for patients because when they stop taking the medications too soon, the infection can spring back with deadly consequences. The bacteria that cause tuberculosis initially infect immune cells in the lungs called alveolar macrophages, which respond by revving up their production of oxygen-containing molecules called reactive oxygen species. However, this defensive response not only harms the bacteria, but also the cell itself through a process called lipid peroxidation, a sort of cellular rust that degrades cell membranes and causes the macrophages to undergo a form of cell death called necrosis. The mass death of infected cells causes damaging inflammation in the lungs and allows the bacteria to spread to other parts of the body, leading to a life-threatening systemic infection.
As complicated as this series of events is, it presents some clear points where intervention could short-circuit the chain reaction. In the new IRP study, Dr. Amaral and his colleagues specifically focused on the initial death of the alveolar macrophages due to necrosis.

Dr. Eduardo Amaral
“When cells cannot repair their membranes, they explode, releasing the bacteria outside the cell,” he explains. “Preventing necrosis helps to control bacterial spread and infection.”
Prior research led by Dr. Amaral found that macrophages from tuberculosis-infected mice had less of an enzyme that helps prevent lipid peroxidation, called Gpx4, as well as less glutathione, a substance Gpx4 needs to do its job.2 Those findings suggested that a lack of Gpx4’s protection against lipid peroxidation contributes to the death of tuberculosis-infected cells, so the new study first aimed to confirm those observations in humans.
Just like in mice, the new study found, immune cells taken from tuberculosis patients had higher levels of lipid peroxidation and lower production of Gpx4 compared to cells from healthy participants. The patients’ blood also had lower levels of glutathione. Furthermore, patients who had less Gpx4 and glutathione and whose cells showed signs of more lipid peroxidation tended to have more severe disease.
“This suggests maybe Gpx4 levels are related to necrosis in patients,” Dr. Amaral says. “That’s why we really think that Gpx4 might be important, so we thought we can try to manipulate Gpx4 to investigate its importance in controlling necrosis.”
Indeed, the IRP researchers found that dampening production of Gpx4 in tuberculosis-infected mice increased lipid peroxidation in immune cells within the animals’ lungs, leading to more necrosis and a higher number of bacteria in the lungs and spleen. On the other hand, boosting Gpx4 production in infected mice reduced lipid peroxidation, necrosis, and the amount of bacteria in those two organs.

Therapies that boost the activity of Gpx4 could enhance the effectiveness of the antibiotics currently used to treat tuberculosis.
If augmenting the production or activity of Gpx4 in mice’s cells helps them fend off tuberculosis, it may be possible to similarly combat the disease in humans by boosting levels of the enzyme or the compounds like glutathione that Gpx4 requires to protect cells from lipid peroxidation. What’s more, measuring the amount of Gpx4 or glutathione in patients’ bodies via a simple blood test could help doctors better predict their patients’ risk for experiencing severe symptoms from tuberculosis infection.
“With that information, we can know if we need to give a patient treatment to boost those levels in order to get a better outcome for antibiotic treatment,” Dr. Amaral says. “If we can find a way to improve the ability of Gpx4 to respond to lipid peroxidation, we’ll be able to better control the detrimental effects of tuberculosis infection.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Amaral EP, Foreman TW, Namasivayam S, Hilligan KL, Kauffman KD, Barbosa Bomfim CC, Costa DL, Barreto-Duarte B, Gurgel-Rocha C, Santana MF, Cordeiro-Santos M, Du Bruyn E, Riou C, Aberman K, Wilkinson RJ, Barber DL, Mayer-Barber KD, Andrade BB, Sher A. GPX4 regulates cellular necrosis and host resistance in Mycobacterium tuberculosis infection. J. Exp. Med. 2022 Nov 7;219(11):e20220504. doi: 10.1084/jem.20220504.
[2] Amaral, EP, Costa S, Namasiyayam S, Riteau N, Kamenyeya O, Mittereder L, Mayer-Barber KD, Andrade BB, Sher A. A major role for ferroptosis in Mycobacterium tuberculosis-induced cell death and tissue necrosis. J. Exp. Med. 2019 Mar 4;216(3):556-570. doi: 10.1084/jem.20181776.
Related Blog Posts
This page was last updated on Wednesday, May 24, 2023
