IRP’s Carolina Barillas-Mury Elected to National Academy of Medicine
NIH Researcher Recognized for Important Insights Into Malaria Transmission

IRP Distinguished Investigator Carolina Barillas-Mury was elected to the National Academy of Medicine in 2021 for her game-changing discoveries about how malaria-causing parasites interact with the mosquito immune system.
In many parts of the world, the high-pitched buzz of a mosquito is a harbinger of more than an annoying itch — it’s a warning of possible malaria infection. Malaria, a disease spread by mosquitos that causes high fever and flu-like illness, is a serious risk for nearly half of the world’s population. According to the World Health Organization, there were 241 million cases of malaria and 627,000 deaths in 2020 alone. More than 95 percent of them occurred in Africa.
Efforts to combat malaria using measures like preventative treatments and environmental mitigation have helped to reduce infections and deaths over the past decade, but those improvements have recently plateaued, according to IRP Distinguished Investigator Carolina Barillas-Mury, M.D., Ph.D., section chief in the Laboratory of Malaria and Vector Research in the National Institute of Allergy and Infectious Diseases (NIAID).
“Although we’ve reduced deaths from malaria by about half, the progress has stalled,” Dr. Barillas-Mury explains. “We’re seeing some regression because insects are becoming more resistant to the insecticides used in bed nets and the malaria parasite itself has become resistant to drugs used to treat the infections.”
Dr. Barillas-Mury’s research on mosquitos and malaria suggests a different approach may one day help control this scourge. In 2021, she was elected to the National Academy of Medicine (NAM) in recognition of her work showing how the Plasmodium parasites that cause malaria slip past the mosquito immune system and how this process helps to maintain malaria transmission.
Malaria is spread when mosquitos feed on a person or animal whose blood contains the Plasmodium parasite. The parasites enter the mosquito’s gut, reproduce, and are transferred to their next host via the infected mosquito’s bite. In regions where the disease is typically found, this cycle is unrelenting. People may receive more than 300 bites from infected mosquitos in a season and, even with periods of treatment, experience three separate malaria infections in a year.

Despite the increasing use and high effectiveness of bed nets in regions where malaria is common, additional tools are needed to reduce deaths from the disease.
“That’s 300 percent coverage — everyone gets infected three times,” Dr. Barillas-Mury says. “Even if you treat a person, they’ll be fine for two or three weeks and then once they stop taking medicine, they get infected again. And the mosquitos will bite several people in a household, so the cycle is very difficult to break.”
Dr. Barillas-Mury and her colleagues have focused on understanding how and why malaria transmission is so efficient. In examining the infection and transmission cycle in mouse models, her lab and others have observed that mosquitos actually do have an immune response that can detect the malaria parasite in their bodies and stop infection. In fact, that system can offer very effective protection, killing as much as 80 percent of the parasites. But therein lies a paradox: it remained a mystery how malaria spreads so easily despite the mosquito’s powerful immune response against the parasite responsible for the disease.
The answer, Dr. Barillas-Mury’s research team found, has to do with geography. In studies focused on Plasmodium falciparum, the most lethal of the malaria-causing parasites, her lab found that when a mosquito is exposed to some varieties of the parasite from foreign locales, the insect’s immune system kicks into action. However, home-grown parasites pass through into the mosquito’s system unnoticed.
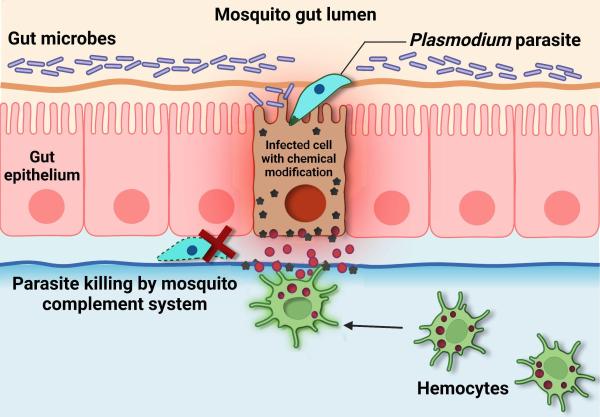
Image by Banhisikha Saha
This diagram shows how the mosquito immune system eliminates the parasites that cause malaria. Once detected, the parasites are killed by a part of the immune system called the complement system.
Normally, when a mosquito bites an infected person or animal, the ingested parasites pass through the wall of the mosquito’s gut and damage the cells they invade. As they are dying, the cells make modifications to the surface of the gut and send out a distress signal, which attracts immune cells called hemocytes. When hemocytes detect the chemical modifications on the gut’s surface caused by the infected cells, they stimulate a response by the mosquito’s body that kills the parasites. However, malaria parasites that evolved in the same location as the mosquitos they infect have developed adaptations that prevent infected cells from making the chemical modifications to the gut that they normally would. This allows malaria parasites to hide from hemocytes and avoid elimination.
Dr. Barillas-Mury also discovered that these adaptions rely on a parasite gene called Pfs47. This gene produces a protein found on the surface of Plasmodium falciparum that renders it invisible to the mosquito’s immune system.
“You can think of it like a key that interacts with a lock-like receptor in the mosquito and turns off the mosquito detection system,” she says. “There are different versions of the receptor protein — the lock — depending on the mosquito species present on different continents. There are also different versions of the parasite’s Pfs47 gene. Those parasites that have a ‘key’ compatible with a certain mosquito receptor can evade the mosquito’s system and spread locally.”
Dr. Barillas-Mury hopes her discoveries can eventually be used to create a vaccine that will coax our bodies to produce molecules called antibodies that can attach to the protein produced by the Pfs47 gene. Binding of these antibodies to the parasite would prevent uninfected mosquitos from picking it up from an infected person and transferring it to an uninfected family member or neighbor. By breaking the cycle of transmission between mosquitos and humans, the vaccine would significantly reduce malaria cases.

Photo courtesy of Jose Luis Ramirez
A vaccine based on Dr. Barillas-Mury’s discoveries could drive down malaria cases by preventing mosquitos from transferring Plasmodium parasites between infected and uninfected people.
Dr. Barillas-Mury’s path toward her potentially game-changing discoveries began when she left Guatemala, where she had earned degrees in biology and medicine, to begin a doctoral program in insect biology at the University of Arizona in 1987. She completed her postdoctoral research at Harvard University and at the European Molecular Biology Laboratory (EMBL) in Heidelberg, Germany. She was well on her way to earning tenure at Colorado State University when the opportunity to come to the National Institutes of Health arose in 2003.
“The position at NIH was unique because they wanted someone to serve as a bridge between insect labs and malaria labs,” she says. “I understood both perspectives, the mosquito ‘vector’ and the parasite, as well as the medical aspects. I feel that my medical training taught me how to think of the mosquito as a patient and to look at how the different systems are all integrated. This has been very useful in how my team approaches questions.”
“It’s been a great journey,” she continues. “I mean, I never saw, coming from Guatemala, that I will work at NIH, much less that I was going to be in the National Academy of Science and now the National Academy of Medicine, so I think I made the right choice sticking to tropical medicine and the medical aspects of molecular biology.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
