Research Briefs
Read about scientific advances and discoveries by NIH intramural scientists: AI-assisted retinal imaging; gene therapy slows pediatric neuropathy; proteins enable immune cells to locate specific microbes; targeting the dark side of an influenza virus protein; multi-omic profiling of lymphoma tumors; mystery enzyme that breaks down bilirubin identified
NEI: AI-ASSISTED RETINAL IMAGING SAVES TIME, IMPROVES RESOLUTION
CREDIT: VINEETA DAS, NEI
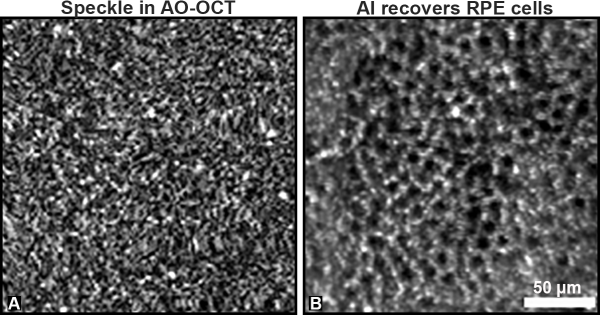
A) AO-OCT provides a more detailed image of the RPE layer, but the cells are obscured by speckle. B) There is a remarkable improvement in RPE cell visualization gained by applying AI to the speckled AO-OCT image. Each dark area represents a single RPE cell.
Clinicians routinely image retinal pigment epithelial cells (RPE), which are crucial to maintaining vision, to detect and treat eye diseases. A recent study found that combining AI with one of those imaging methods, adaptive-optics optical-coherence tomography (AO-OCT), made the process 100 times as fast and improved image contrast 3.5-fold compared with previous methods.
AO-OCT is a powerful tool that can produce 3D retinal images at the cellular scale; however, the imaging process creates noise known as speckle, which necessitates that hundreds of images be manually averaged together to improve contrast.
A team led by Vineeta Das and Johnny Tam in NEI’s Clinical and Translational Imaging Section created a deep learning algorithm that efficiently despeckled a single image capture comparably with the manual method while also improving contrast. “Thinking about AI as a part of the overall imaging system, as opposed to a tool that is only applied after images have been captured, is a paradigm shift for the field of AI,” said Tam in a press release. (NIH authors: V. Das, F. Zhang, A.J. Bower, J. Li, T. Liu, N. Aguilera, B. Alvisio, and J. Tam, PMID: 38600290)
Vineeta Das, NEI Clinical and Translational Imaging Section, explains how artificial intelligence improves imaging of the eye’s light-sensing retina.
NINDS, CC, NEI, NIAID: INTRATHECAL GENE THERAPY SLOWS PEDIATRIC NEUROPATHY SYMPTOMS
CREDIT: BONNEMANN LAB, NINDS
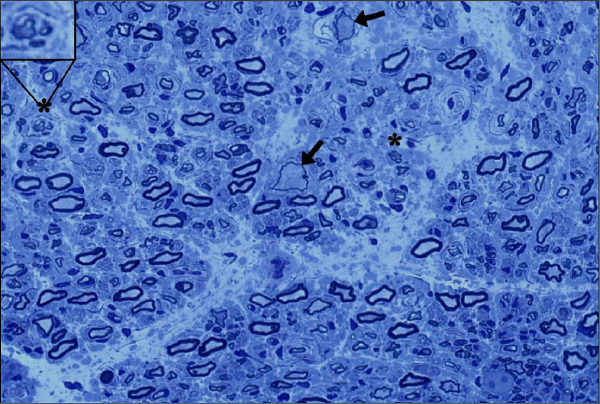
Biopsy of a sensory nerve from a participant in the GAN gene therapy trial (arrows indicate giant axons; regenerating nerve cluster in upper left).
A first-in-human intrathecal gene therapy for the treatment of giant axonal neuropathy (GAN) in children is able to slow symptom progression, according to a recent collaborative NIH study.
GAN is a rare, inherited, and rapidly progressive neurodegenerative disorder caused by the absence or loss of function of gigaxonin. The disease is typically fatal by 30 years of age, and symptoms typically begin as sensory and motor deficits occurring as early as two years old.
In this study, clinicians administered an adeno-associated virus containing a functional GAN gene intrathecally, that is, by injection into the spinal fluid.
The investigators administered one of four intrathecal doses of the therapy to 14 pediatric participants who had also been enrolled in a natural history study at the NIH. Among the various dose groups, motor function scores at one year showed the treatment seemed to slow the rate of motor decline, and six participants regained some sensory nerve activity. The treatment was generally well tolerated with one serious adverse event, a fever linked to the therapy.
The authors plan to continue dosing with the investigational therapy for the treatment of GAN and note that the study might inform other gene therapies that could be delivered intrathecally. (NIH authors: D.X. Bharucha-Goebel, J.J. Todd, D. Saade, A.R. Foley, P. Mohassel, G. Norato, T. Lehky, J.D. Heiss, M. Jain, M. Waite, W.M. Zein, L.A. Huryn, E.M. Kang, and C.G. Bönnemann, PMID: 385077652)
[BY JOHN CARLO JADORMEO COMBISTA, NIMH]
NIA: TWO PROTEINS COLLABORATE TO MOUNT A PATHOGEN-TAILORED IMMUNE RESPONSE
NIA scientists found that the coordinated actions of two inflammatory signaling proteins, RelA and c-Rel, enable immune cells to determine the presence of specific microbes before engulfing them. RelA and c-Rel help these immune cells, called macrophages, distinguish the microbes via their shed pieces, known as ligands. This action produces a response appropriate for the specific pathogen. These two proteins are important components of the nuclear factor kappa B (NF-kappa-B) signaling pathway, which is responsible for regulating the immune response of a mouse or human to pathogens or cellular damage. NF-kappa-B is also broadly involved in all inflammatory conditions.
The research team led by Myong-Hee “Mia” Sung, an NIA tenure-track investigator, made the discovery by monitoring live macrophages from fluorescent double-knockin reporter mice the group had previously generated for quantitative single-cell studies. The live-cell imaging showed that RelA and c-Rel conveyed more information about ligands than either subunit alone. Based on this signaling information, Sung and colleagues used machine learning to predict to which microbial ligand macrophages were binding so that they could mount an immune response against a pathogen.
The authors state that the coordinated signaling between RelA and c-Rel may have developed during evolution, and their finding provides insight into how NF-kappa-B proteins mediate a wide range of microenvironmental cues in immune cell communication. (NIH authors: S.M.T. Rahman, M. Aqdas, and M.H. Sung, PMID: 38483906)
[BY ROBIN ARNETTE, NIA]
NIAID: “DARK SIDE” OF INFLUENZA VIRUS PROTEIN OFFERS NEW TARGET

CREDIT: NIAID
Colorized transmission electron micrograph of influenza A virus particles
A new flu-fighting target was uncovered by a research team at NIAID’s Vaccine Research Center. Because influenza mutates and generates new strains, there is a constant need to develop new vaccines that remain effective against the rapidly evolving virus.
Investigators targeted a conserved region of a glycoprotein called neuraminidase (NA), an essential surface protein of the influenza virus. The underside of the NA protein head, termed the “dark side,” is believed to be hidden from the immune system and thus understudied in influenza research. However, the region is less susceptible to mutations and remains relatively unchanged between different virus strains.
The scientists isolated human antibodies against viral epitopes—part of the viral protein which can be recognized by the host immune system—located within the dark side region of NA from people who recovered from influenza infection. These antibodies were able to prevent lethal infection in mice when administered before and after exposure to H3N2 influenza.
Cryogenic electron-microscopy analysis of the antibodies bound to NA revealed that they targeted nonoverlapping epitopes of the dark side region, suggesting that targeting that area could be helpful in designing broadly protective influenza vaccines. (NIH authors: J. Lederhofer, Y. Tsybovsky, L. Nguyen, J.E. Raab, A. Creanga, T. Stephens, R.A. Gillespie, H.Z. Syeda, B.E. Fisher, M. Skertic, C. Yap, A.J. Schaub, R. Rawi, P.D. Kwong, B.S. Graham, A.B. McDermott, S.F. Andrews, and M. Kanekiyo, PMID: 38430907)
[BY DEVIKA BOSE, NEI]
NIAID, NCI: MULTI-OMIC PROFILING OF FOLLICULAR LYMPHOMA TUMORS
Lymph nodes are one of the most diverse sites for cancer and metastasis. Located throughout the body, they are connected to many organ systems via lymphatic circulation. In some cases, B cells housed in lymph nodes can become malignant, resulting in B-cell lymphoma. A research team led by NIAID’s Andrea Radtke and NCI collaborators took a deep dive into a generally incurable form of this disease, called follicular lymphoma (FL), and uncovered some of the genomic, transcriptional, and proteomic factors that contribute to the diverse clinical outcomes observed among FL patients.
Within the tumor environment of FL patient samples, the investigators found enhanced cell signaling and changes in the stromal cells that make up the connective tissue of tumors. The researchers designed an approach that integrated single-cell RNA sequencing and IBEX (iterative bleaching extends multiplexity) imaging, which is an advanced spatial imaging method developed by NIAID investigators that can analyze multiple targets with immunolabeling. This analysis revealed more stromal and myeloid cells than in previous studies in which the tumor was not left intact.
The researchers then analyzed FL samples from patients who had early relapse of disease after beginning therapy. Changes in those samples included further transcriptional differences in B cell receptor signaling, extracellular matrix remodeling, and glutaminergic receptor activities that can drive tumor growth. Furthermore, distinct architectural changes in the lymph node follicles were found in patients 20 months prior to first progression and relapse.
According to the authors, this study provides a key insight into FL biology and may help physicians and researchers predict disease behavior and improve patient outcomes. (NIH authors: A.J. Radtke, Z. Yaniv, B.C. Lowekamp, E. Speranza, S. Pittaluga, A.L. Shaffer 3rd, J.D. Phelan, T. Davies-Hill, D.W. Huang, M. Kelly, J. Muppidi, J.L. Davis, J.M. Hernandez, W.H. Wilson, E.S. Jaffe, L.M. Staudt, M. Roschewski, and R.N. Germain, PMID: 38428410)
[BY STEPHEN ANDREWS, NCI]
NLM, NICHD: MYSTERY SOLVED AS RESEARCHERS IDENTIFY KEY ENZYME THAT BREAKS DOWN BILIRUBIN
CREDIT: NLM
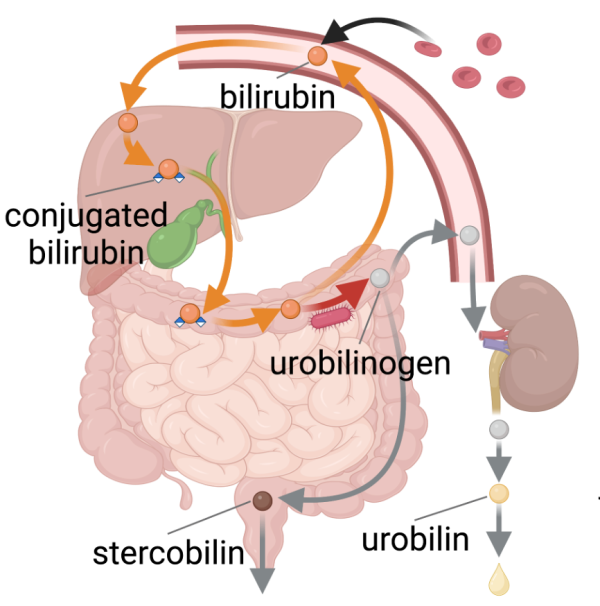
Researchers recently discovered bilirubin reductase, a key enzyme involved in the production of urobilinogen.
The complete process of breaking hemoglobin down to urobilin is essential for clearing our bodies of cellular waste, and issues with this breakdown process can lead to health problems such as jaundice. While much of this process was already well understood, the identity of the enzyme that turns bilirubin into urobilinogen remained a mystery.
That is, until recently. Xiaofang Jiang, a principal investigator at NLM, and her collaborators used a combination of modern comparative genomics analyses and novel biochemical assays to identify bilirubin reductase, a key enzyme in the human gut microbiome that is responsible for the bilirubin-to-urobilinogen reduction process.
After this discovery, data were analyzed from previous studies and found that while healthy adults almost always had this enzyme, it was often missing in infants who were susceptible to jaundice as well as over 30% of adults with inflammatory bowel disease.
“Now that we’ve identified this enzyme, we can start investigating how the bacteria in our gut impact circulating bilirubin levels and related health conditions,” said Jiang. And because the gut microbiome has been linked to other diseases and conditions, these findings can potentially guide future work to understand the gut microbiome’s role in human health even better. According to Jiang, what they’ve discovered is “just the tip of the iceberg.” (NIH authors: K. Dufault-Thompson, A. Zhong, and X. Jiang, PMID: 38172624)
To read more (and watch a video) about these research findings, visit NLM Musings from the Mezzanine.
[BY FELICITY FOX, NLM]
This page was last updated on Tuesday, December 3, 2024
