Research Briefs
Read about scientific advances and discoveries by NIH intramural scientists: weaker immune response in children with mitochondrial disorders; tumor cell’s location and environment affect its identity; primitive sea creature provides clues to aging and healing; proteins in plasma portend risk of dementia; women treated for breast cancer may age faster; cells preparing for mitosis can reverse their decision to divide.
NHGRI, CC: WEAKER IMMUNE RESPONSE TO VIRAL INFECTIONS IN CHILDREN WITH MITOCHONDRIAL DISORDERS
CREDIT: NHGRI
Mitochondria convert food and oxygen into energy. Genomic variants in more than 350 genes have been linked to mitochondrial disorders.
Children with mitochondrial disorders (MtD) may have difficulty fending off and recovering from viral infections, according to a study led by NHGRI scientists. The study was one of the first to investigate how B cells, which produce antibodies in response to viral infections, are affected in human MtD.
The investigators performed single-cell RNA sequencing in peripheral blood cells of children with MtD to analyze gene activity and found biomarkers of cellular stress directly related to mitochondrial dysfunction. “We think that B cells in these patients undergo cellular stress when they turn into plasma cells and produce antibodies,” said senior author Peter McGuire. “The B cells are too fragile due to their limited energy, so they are unable to survive the stressful conditions.”
The authors noted that their findings have broad implications not only for understanding the role of mitochondrial dysfunction in the human immune response, but also for finding targeted treatments for children with MtD, who have weaker immune responses and are at risk for life-threatening infections. (NIH authors: E.M. Gordon-Lipkin, P. Banerjee, J.L.M. Franco, T. Tarasenko, S. Kruk, E. Thompson, D.E. Gildea, S. Zhang, T.G. Wolfsberg, NISC Comparative Sequencing Program, W.A. Fiegel, and P.J. McGuire, Front Immunol 14:1142634, 2023; DOI 10.3389/fimmu.2023.1142634)
[BY: SEPPIDEH SAMI, CC]
NCATS, NCI, CC: TUMOR CELL’S LOCATION AND ENVIRONMENT AFFECT ITS IDENTITY
CREDIT: DAVID B. MORSE, PH.D., HARVARD
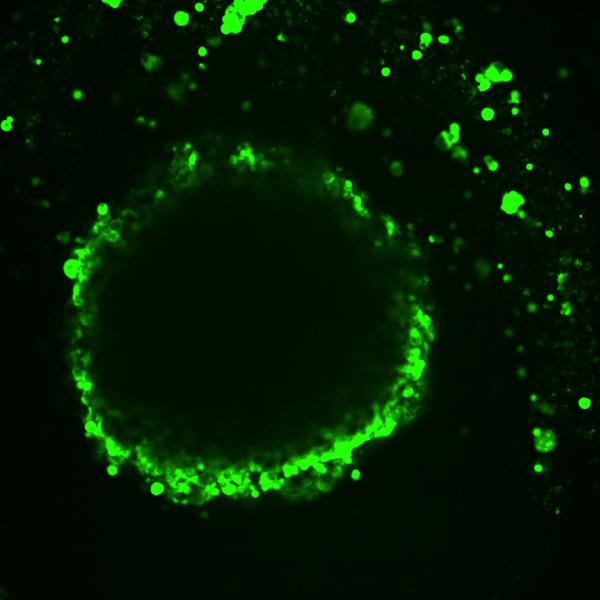
Image shows differences in cell layers’ fluorescent-dye uptake in a model of high-grade serous ovarian cancer.
A cell’s location and environment within a cancerous tumor can strongly influence which genes are active, according to a new study co-led by NCATS scientists. To explore that concept, Craig Thomas, Leader of NCATS' Chemistry Technology group, and his colleagues developed a new system that pairs a fluorescent dye with the ability to unravel an individual tumor cell’s genetic activity. “Two cells can be genetically identical but have different cellular identities, meaning different genes are turned on because of their location and environment,” said Thomas.
Some of their findings confirmed old suspicions. Others provided new insights: With the help of 3D models of ovarian cancer tumors, the researchers found that gene activity in cells at or near a tumor’s surface differed from that of cells closer to the tumor center. They also found evidence of metastatic activity on the surface and of cancer cells finding ways to work against the immune system.
By better understanding how tumors are structured, clinicians could potentially identify treatment strategies focused on specific areas in tumors, which could lead to improved therapies for cancers, neurological disorders, and other site-dependent diseases. (NIH authors: D.B. Morse, A.M. Michalowski, M. Ceribelli, D. Riley, T. Davies-Hill, T. Voss, S. Pittaluga, J. Liu, and C.J. Thomas, Cell Syst 14:464-481.E7, 2023; DOI:10.1016/j.cels.2023.05.003)
[BY STEVEN BENOWITZ]
NHGRI: PRIMITIVE SEA CREATURE PROVIDES CLUES TO AGING AND HEALING

CREDIT: MADDISON HARMAN, WHITNEY MARINE LABS
Hydractinia symbiolongicarpus, a small, tube-shaped animal that lives on the shells of hermit crabs, is capable of entirely regenerating itself by reverting differentiated cells back into stem cells when the organism is wounded.
NHGRI scientists and their colleagues demonstrated how a tiny sea creature, Hydractinia symbiolongicarpus, is capable of entirely regenerating itself by reverting differentiated cells back into stem cells when the organism is wounded. This finding provides insight into cellular signaling pathways that might similarly promote healing in more complex animals, including humans.
Hydractinia are made up of a long trunk with a head and mouth at its tip. When the head is separated from the trunk, it is able to regrow a brand-new body with the help of highly versatile cells called i-cells. In this new study, the investigators found that although stem cells are absent in the heads of uninjured animals, the cells reappeared in the separated head tissue and were able to drive regeneration.
The research team used RNA sequencing to identify several genes activated in head tissue adjacent to the injury—genes typically associated in other organisms with an aging-related process called senescence. When the scientists deleted those genes, the modified animals were unable to produce the stem cells necessary for tissue regeneration and died when their heads were separated from their trunks. “We still don’t understand how senescent cells trigger regeneration or how widespread this process is in the animal kingdom,” said study author Andy Baxevanis. “Fortunately, by studying some of our most distant animal relatives, we can start to unravel some of the secrets of regeneration and aging.” (NIH author: A.D. Baxevanis, Cell Rep 42:112687, 2023; DOI: 10.1016/j.celrep.2023.112687)
[BY CODY R.K. CONRAD, NIAID]
NIA, NINDS: PROTEINS IN PLASMA PORTEND RISK OF DEMENTIA
Scientists from NIA and NINDS and their colleagues have discovered that several proteins present in plasma at middle age are linked to developing dementia later in life.
In order to better understand the association of proteins with such a protracted disease, plasma samples from 10,981 middle-aged participants were analyzed over a 25-year period. Analysis of 4,877 proteins revealed that 32 were associated with increased dementia risk, after adjustment to rule out other contributing factors.
The investigators discovered that near-term dementias were linked to distinct biological pathways compared with long-term dementias. One protein, GDF15, was found to have a particularly strong association with all stages of the disease. And analysis of an immune pathway revealed that the protein SERPINA3 may contribute to the development of Alzheimer’s disease (AD).
Finally, a machine-learning analysis demonstrated that predicting future dementias using the identified dementia-associated plasma proteins was significantly improved when assessed with other contributing factors. This research highlights the need for additional study into AD and dementia pathways outside of the well-known amyloid, tau, and neurodegenerative mechanisms. (NIH authors: K. Walker, M. Duggan, Z. Peng, and R. Gottesman, Sci Transl Med 2023; DOI:10.1126/scitranslmed.adf5681)
[BY CODY R.K. CONRAD, NIAID]
NIEHS: WOMEN TREATED FOR BREAST CANCER MAY AGE FASTER THAN CANCER-FREE WOMEN
Past studies suggested that women who overcome breast cancer are at elevated risk for age-related disease. But biological aging, or the general health of your body’s cells, can be difficult to define. In a recent study, NIEHS investigators explored this concept further by tracking naturally occurring chemical modifications made to DNA, called DNA methylation. Small variations in methylation are linked to an increased incidence of age-related illness and mortality risk.
The scientists examined methylation changes by drawing blood from 417 women over eight years. Half of the women developed and were treated for breast cancer during that time, and the other half remained cancer-free. Blood samples were examined using three different “methylation clocks” to determine the effect of breast cancer on biological aging.
The results showed that women treated for breast cancer had accelerated biological ages across all three methylation clocks compared with their cancer-free counterparts. When sorted by treatment type, the researchers found that surgery had little effect, but that radiation therapy was strongly associated with faster biological aging, even after controlling for other factors such as race and tumor type.
While these findings shed some light on how breast cancer can increase risk of age-related disease, the researchers noted that modern radiation therapy remains effective and that patients should discuss all possible treatment options with their doctors. (NIH authors: J.K. Kresovich, K.M. O’Brien, Z. Xu, C.R. Weinberg, D.P. Sandler, and J.A. Taylor, J Natl Cancer Inst 2023; DOI:10.1093/jnci/djad117)
[BY JONATHAN CHU, NIAID]
NCI: CELLS PREPARING FOR MITOSIS CAN REVERSE THEIR DECISION TO DIVIDE
CREDIT: NCI
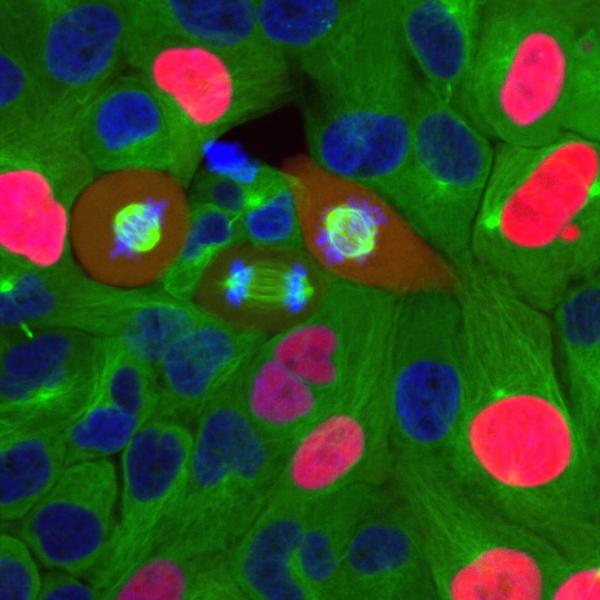
Breast cancer cells going through the cell cycle.
In cancer, the cell cycle becomes dysregulated when cancer cells enter the cycle and undergo the process of mitosis at a greater rate than normal cells. NCI researchers led by Stadtdman Investigator Steven Cappell recently published new research in Nature that found cells preparing to divide can reverse that decision, calling into question long-held beliefs about cell division.
When cells receive growth-promoting signals, called mitogens, they enter the cell cycle and prepare to divide. Scientists have traditionally thought that beyond a certain point known as the restriction point, a positive feedback loop between mitogen signaling and proteins that govern cell division led to an irreversible progression of the cycle toward mitosis. However, Cappell’s group showed that upon loss of mitogen signaling, some cells could return to a resting state even after reaching the restriction point.
The researchers found that cell-cycle regulators CDK4 and CDK6 promoted synthesis of the protein cyclin A2, which in turn induced cell division. In experiments with different kinds of cells, repressing that process at any time before mitosis allowed cells to exit the cell cycle and halt mitosis.
These findings point toward a new model that is more dynamic and reversible and may inform future cancer therapies that target cell division. (NIH authors: J.A. Cornwell, A. Crncec, M.M Afifi, K. Tang, R. Amin, and S.D. Cappell, Nature 619:363–370, 2023)
[BY STEPHEN ANDREWS, NCI]
This page was last updated on Thursday, September 7, 2023
