COVID-19 Timeline at NIH
Highlights Through April 30, 2020
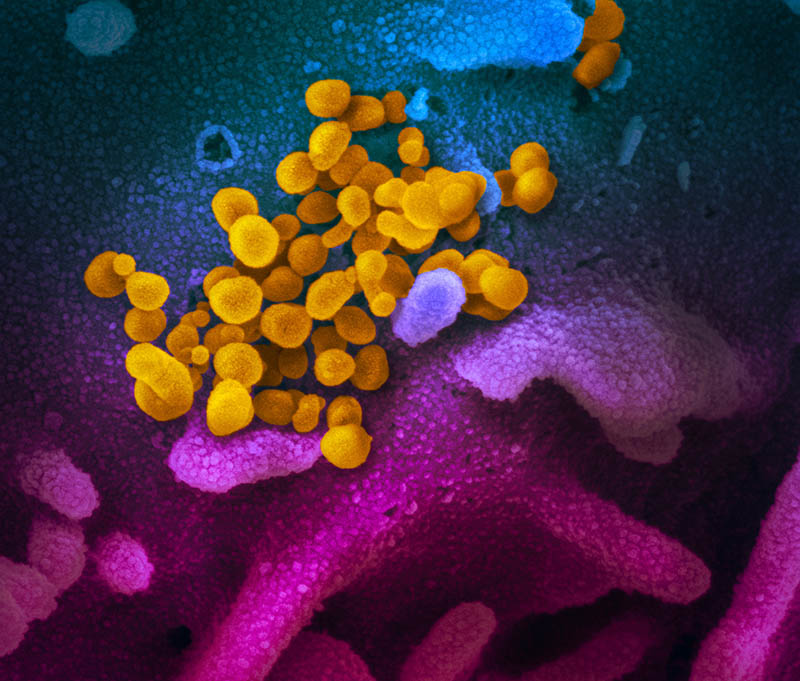
CREDIT: ROCKY MOUNTAIN LABORATORIES, NIAID
This scanning electron microscope image shows SARS-CoV-2 (yellow)—the virus that causes COVID-19—isolated from a patient in the United States. The virus is emerging from the surface of cells (blue and pink) cultured in the lab. The image was captured and colorized at NIAID’s Rocky Mountain Laboratories in Hamilton, Montana.
The COVID-19 pandemic has certainly upended the way work is getting done at NIH these days. Thousands of NIHers are teleworking, and all labs have minimal staffing to carry on essential research or maintenance operations. But many NIH scientists have been working around the clock trying to decipher the secrets held by the virus that causes the disease and to develop treatments and vaccines. The NIH Catalyst has compiled a timeline highlighting much of what is going on during this historic time (see page 11).
January 23: NIH officials discuss novel coronavirus that recently emerged in China.
January 29: President Donald Trump announces the formation of the Coronavirus Task Force, which includes HHS Secretary Alex Azar and NIAID Director Anthony Fauci; On February 27, NIH alum Deborah Birx, Ambassador-at-Large and United States Global AIDS Coordinator, was appointed to the task force. (She completed two fellowships in Fauci’s lab from 1983 to 1986).
February 13: NIAID researchers publish paper in the Proceedings of the National Academy of Sciences showing that the experimental antiviral remdesivir prevents MERS coronavirus disease in monkeys. (Proc Natl Acad Sci USA 2019; DOI:10.1073/pnas.1922083117)
Feb 25: The NIAID-sponsored first clinical trial—Adaptive COVID-19 Treatment Trial (ACTT)—of remdesivir to treat COVID-19 begins at the University of Nebraska Medical Center in Omaha.
February 28: NIH’s Anthony Fauci and Clifford Lane and CDC Director Robert Redfield publish a commentary in the New England Journal of Medicine on “COVID-19: Navigating the uncharted.” (N Engl J Med 382:1268–1269, 2020; DOI:10.1056/NEJMe2002387)
March 3: President Trump visits NIAID’s Vaccine Research Center to discuss coronavirus.
March 5: Maryland declares a state of emergency and catastrophic health emergency to control and prevent the spread of COVID-19 within the state.
March 6: NIH launches its Coronavirus Response Team, which begins meeting daily. It is co-chaired by NIH Principal Deputy Director Lawrence Tabak and NIH Director for Management Alfred Johnson.
March 10: Clinical Center starts deferring elective patients—both inpatients and outpatients.
March 11: NIH announces temporary suspension of weekly community markets, food trucks, and Recreation and Welfare Association (R&W) visiting merchants located in Buildings 10 and 31.
March 12: Clinical Center starts screening patients and visitors coming into Building 10.
March 15: NIH reports first known employee with COVID-19 infection.
March 16: Most NIH employees and trainees begin teleworking. Patient-care providers at the Clinical Center, animal-care workers, people working directly on COVID-19 research, and certain other workers are exempted. Telework was to be through April 3, but it was extended to May 1, and then extended again through May 31.
March 16: Closure of all schools in Maryland, Virginia, and Washington, D.C., begins.
March 16: An NIH-funded clinical trial of an NIAID-developed investigational vaccine for COVID-19 begins at Kaiser Permanente Washington Health Research Institute in Seattle.
March 17: NIH establishes large-scale, centralized COVID-19 screening (and testing, if needed) for employees.
March 17: Scientists from NIAID’s Rocky Mountain Laboratories (Hamilton, Montana), CDC, the University of California at Los Angeles, and Princeton University report that the virus that causes COVID-19 is stable for several hours to days in aerosols and on surfaces. (N Engl J Med 382:1564–1567, 2020; DOI:10.1056/NEJMc2004973)
March 18: Changes to NIH campus services are announced and include the closing of the NIH Library (services and resources still available online), some cafeterias, coffee bars, concession stands, and fitness centers (closed on March 16). Throughout the month, there are reductions in other services. The NIH childcare centers remain open for the children of essential employees.
March 18: A new COVID-19 Scientific Interest Group is established. The group aims to keep the NIH community aware of resources, including funding opportunities, to support research on COVID-19 and SARS-CoV-2. In addition to hosting a LISTSERV email newsletter, the group also organized a virtual lecture series to temporarily replace the Wednesday Afternoon Lecture Series. The first lecture, held on April 15, featured Hillary Marston (NIAID), who talked about “The Biomedical Research Response to COVID-19: A View from NIAID.” As of April 30, 2020, the group has about 1,300 members.
March 20: NIH shifts non-mission-critical laboratory operations to minimal maintenance phase to further reduce the transmission of COVID-19 and further enhance the safety of staff.
March 20: NIH holds its first virtual town hall meeting on the coronavirus response. More than 24,000 have watched the broadcast. (NIH only: https://videocast.nih.gov/watch=36221)
March 23: NIH announces the launch of a website and a COVID-19 virtual–training initiative for frontline responders including emergency medical personnel, firefighters, law enforcement officers, environmental cleanup workers, high-risk custodial service workers, food processing and delivery workers, water and sewage treatment workers, sanitation workers, and health–care facility employees.
March 23: Maryland orders that large gatherings (greater than 10 people) and events be prohibited, senior centers be closed, and all nonessential businesses and other establishments be closed.
March 24: Clinical Center enrolls first two participants in NIAID remdesivir trial.
March 23: Additional restrictions are imposed on Clinical Center visitors.
March 25: The National Library of Medicine expands access to coronavirus literature through PubMed Central.
March 26: Screening of all staff members entering Building 10 begins.
March 27: Emory University in Atlanta site is added to NIAID-supported clinical trial of the NIAID-developed investigational vaccine for COVID-19.
March 27: In the Clinical Center, surgical masks begin to be required for everyone doing direct patient care.
March 27: Maryland Governor Larry Hogan mandates that all childcare centers in the state close at the end of the business day.
March 30: NIH-sponsored childcare centers will remain closed; awaiting direction from the state of Maryland as to when they will be allowed to re-open.
March 30–April 1: Stay-at-home orders for Maryland, Virginia, and Washington, D.C., take effect.
April 2: NIDA Director Nora Volkow outlines, in the Annals of Internal Medicine, the potential risks to people who smoke and use drugs during the COVID-19 pandemic. (Ann Intern Med 2020; DOI:10.7326/M20-1212)
April 2: The NIH Clinical Center begins providing surgical masks to everyone who enters the building.
April 9: NHLBI begins a clinical trial at Vanderbilt University Medical Center (Nashville) to evaluate the safety and effectiveness of hydroxychloroquine for the treatment of adults hospitalized with COVID-19.
April 10: NIH Director Francis Collins announces that the NIH Office of Data Science Strategy has compiled COVID-19-related datasets, computational tools, and other resources in one easily accessible website location.
April 10: NIAID begins recruiting people for a serology study to determine how many adults in the United States without a confirmed history of infection with SARS-CoV-2, the virus that causes COVID-19, have antibodies to the virus. The presence of antibodies in the blood indicates a prior infection. The study expects to analyze blood samples from as many as 10,000 volunteers.
April 13: Montgomery County, Maryland, begins to require that all shoppers in the county wear face covering in grocery stores, pharmacies, and other retail establishments.
April 15: A study by investigators at NIAID’s Rocky Mountain Laboratories (Hamilton, Montana) validates decontamination methods for re-use of N95 respirators. (non-peer-reviewed preprint server medRxiv, April 24, 2020; DOI:10.1101/2020.04.11.20062018
April 15: In a letter to the editor of the New England Journal of Medicine, NIDDK investigators report the results of a laser-light-scattering experiment that provided visual evidence of how speech-generated droplets moved through the air and that putting a damp cloth over the mouth decreased the number of droplets.(New Engl J Med 2020; DOI:10.1056/NEJMc2007800)
April 17: NIH and the Foundation for NIH announce that they are launching a public-private partnership—including 16 biopharmaceutical companies, HHS, CDC, FDA, and the European Medicines Agency—to speed COVID-19 vaccine and treatment options. The planned Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership will develop a collaborative framework for prioritizing vaccine and drug candidates, streamlining clinical trials, coordinating regulatory processes, and/or leveraging assets among all partners to rapidly respond to the COVID-19 and future pandemics.
April 17: NIDCR begins enrolling research participants for a study—open to NIH staff and their close contacts only—at the Clinical Center to determine whether SARS-CoV-2, the virus that causes COVID-19, is present in the saliva of asymptomatic individuals and can be transmitted by speaking, and whether wearing a mask will prevent this route of transmission. For protocol details, go to https://clinicalstudies.info.nih.gov/ProtocolDetails.aspx?A_2020-D-0094.html.
April 17: Early treatment with the experimental antiviral drug remdesivir significantly reduces clinical disease and damage to the lungs of rhesus macaques (Macaca mulatta) infected with SARS-CoV-2, the coronavirus that causes COVID-19, according to NIAID scientists. (non-peer-reviewed preprint server bioRxiv, 2020; DOI:10.1101/2020.04.15.043166)
April 17: NIAID scientists report that an investigational vaccine protected two groups of rhesus macaques (Macaca mulatta) from disease caused by Middle East respiratory syndrome coronavirus (MERS-CoV, a relative of the coronavirus that causes COVID-19). The scientists and colleagues are pursuing similar studies with a vaccine candidate against SARS-CoV-2, the coronavirus that causes COVID-19. (non-peer-reviewed preprint server bioRxiv, April 13, 2020; DOI:10.1101/2020.04.13.036293)
April 17: The NIAID-supported clinical trial of an experimental COVID-19 vaccine that started on March 16, begins enrolling older adults. The trial is being conducted in Seattle and Atlanta, and NIAID’s Vaccine Research Center Clinic (Bethesda, Maryland) has been added as a site.
April 21: Expert U.S. panel—including co-chairs H. Clifford Lane (NIAID) and Henry Masur (NIH Clinical Center)—develop NIH treatment guidelines for COVID-19.
April 22: DDIR Michael Gottesman announces the Intramural Targeted Anti-COVID-19 (ITAC) funding program. The program was made possible by NIAID, which has provided generous funding to support COVID-19 research activities.
April 23: The new “NIAID Strategic Plan for COVID-19 Research” provides details for accelerating research to diagnose, prevent, and treat COVID-19. https://www.niaid.nih.gov/sites/default/files/NIAID-COVID-19-Strategic-Plan-2020.pdf.
April 24: NIH held its second virtual town hall meeting to update the NIH community on NIH’s activities in response to the COVID-19 pandemic and to answer questions. More than 21,000 people have watched the broadcast (NIH only: https://videocast.nih.gov/watch=37446)
April 29: NIH announces a new initiative aimed at speeding innovation, development, and commercialization of COVID-19 testing technologies. With a $1.5 billion investment from federal stimulus funding, the newly launched Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding into early innovative technologies to speed development of rapid and widely accessible COVID-19 testing.
April 29: Hospitalized patients with advanced COVID-19 and lung involvement who received remdesivir recovered faster than similar patients who received placebo, according to a preliminary data analysis from a randomized, controlled trial involving 1,063 patients, which began on February 21. The trial (known as the Adaptive COVID-19 Treatment Trial, or ACTT), sponsored by NIAID, is the first clinical trial launched in the United States to evaluate an experimental treatment for COVID-19.
COVID-19 is an emerging, rapidly evolving situation.
- Get the latest public health information from CDC: https://www.coronavirus.gov
- Get the latest research information from NIH: https://www.nih.gov/coronavirus
This page was last updated on Thursday, March 24, 2022
