Discovering a Cure for Sickle-Cell Disease
The Journey of Physician–scientist and Musician John Tisdale, M.D.
If you were to dissect the anatomy of the NIH director’s band (a.k.a. “ARRA” or the “Affordable Rock ‘n’ Roll Act), you would find the supporting beat of John Tisdale’s bass guitar. Although primarily a senior investigator and chief of the Cellular and Molecular Therapeutics Branch in the National Heart, Lung, and Blood Institute (NHLBI), in the National Heart, Lung, and Blood Institute (NHLBI), Tisdale plays a secondary role as the resident bass guitarist for NIH musical ensembles. His skills—of applied creativity, supporting others from the backline, and healing the seemingly unfixable—span his vocations as a physician–scientist and musician and have helped him throughout his journey toward developing a cure for sickle-cell disease (SCD).

CREDIT: LEWIS SCHRAGER
When he’s not conducting research on sickle-cell disease, John Tisdale (second from left) plays bass guitar in the NIH Director’s Band. NIH Director, Francis Collins (fourth from left) is playing the red guitar.
Road to NIH
Tisdale’s path to becoming a physician–scientist began when he was 8 years old, with an unfortunate accident involving a self-propelled lawnmower. While mowing the lawn he stumbled, still squeezing the propelling handle, and accidentally directed the lawnmower toward his leg. He landed in the emergency room with a major knee injury. Physician after physician claimed, “No, I can’t fix that” and “Nope, can’t be done.”
Finally one physician asserted, “Yeah, I think can fix that.” And after several surgeries and physical therapy, Tisdale’s knee began to heal. Slowly, he regained the ability to walk and eventually to run. That physician’s ability to mend the seemingly unfixable inspired Tisdale to become a doctor himself. “We often as human beings try to do things that we see other people do well,” Tisdale said.
As he navigated the path to becoming a physician, music remained a constant in his life. While pursuing his B.A. in chemistry at the College of Charleston (Charleston, South Carolina) and an M.D. from the Medical University of South Carolina (Charleston), he played cover tunes with his friends and fellow band members at local bars and earned enough to help pay his tuition. He decided to do his residency at Vanderbilt University School of Medicine in Nashville partly because he hoped to play his bass guitar in the “Music City” while not at work. But residency was so time consuming that he was forced to place his musical career on perpetual hold.
It was during his residency (1990–1994) that Tisdale first encountered SCD, which was, at the time, a disease without a cure. Here was something seemingly unfixable that he wanted to fix.
SCD, which affects about 100,000 people in the United States and millions worldwide, is an inherited blood disorder caused by a single-nucleotide mutation in the gene encoding the beta chain of the hemoglobin protein. The resulting abnormal hemoglobin gene triggers the production of distorted, sickle-shaped, red blood cells that block circulation and cause anemia, agonizing pain, and organ damage. Most people with SCD have a life expectancy of 42–47 years.
During Tisdale’s residency, the standard treatment for patients with SCD was pain medications. “That was all we had, good pain medicine,” he said. The opportunity to address this issue as a physician–scientist is what brought him to NIH as a hematology fellow in 1994. He became a clinical tenure-track investigator in 1998 and a tenured investigator in 2006. With only a single-nucleotide mutation as the culprit, Tisdale believed SCD to be a curable illness.
Fine-tuning Sickle-Cell Treatments
To Tisdale, both music and science are creative endeavors, with the skillset required of one naturally lending itself to the other. Now, as a senior investigator, Tisdale is embodying that expertise in the laboratory and the clinic to produce curative therapies that cover all SCD patients.
Nowadays, SCD can be treated with two types of bone-marrow stem-cell transplants: allogeneic (from a donor) and autologous (from oneself). In 2009, Tisdale and his colleagues optimized a low-toxicity method of performing allogeneic transplants in which healthy hematopoietic (blood-producing) stem cells from a matching donor (usually a sibling) are infused into the sickle-cell patient. But first, the patient’s immune system had to be suppressed using chemotherapy to destroy the bone-marrow stem cells. The method, however, is too toxic for adults who have had years of accumulated organ damage. Tisdale’s group developed and tested a new regimen that involved only partially replacing the bone marrow and using low-dose whole-body radiation to suppress the immune system and two drugs (alemtuzumab and sirolimus) to prevent rejection of the donor bone marrow. In a study with 10 patients, this new method reversed severe cases of SCD in nine of them, even in instances of lung failure, (N Engl J Med 361:2309–2317, 2009; DOI:10.1056/NEJMoa0904971)
In a 2014 study that included patients from the 2009 work, Tisdale and colleagues showed that their method reversed SCD in nearly all the patients (26 out of 30), and half of them were able to safely stop immunosuppressant medications. (JAMA 312:48–56, 2014; DOI:10.1001/jama.2014.7192)
But only about 10% of SCD patients can find a matched donor, according to Tisdale. Although the best option is for the donor to be a sibling, not all siblings are full matches.
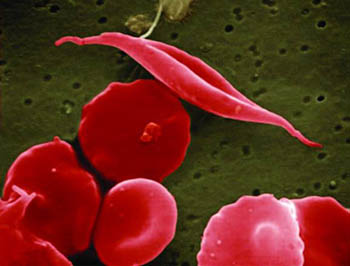
CREDIT: NATIONAL CENTER FOR ADVANCING TRANSLATIONAL SCIENCES
Sickle cells: A defect in hemoglobin causes red blood cells to become rigid and take on a crescent (sickle) shape, blocking small blood vessels and causing decreased blood flow, inflammation, and pain. Shown: Red blood cells; the stretched out one is a sickle cell.
To circumvent the need for a donor, Tisdale’s team is exploring gene therapies for autologous stem-cell transplantations. Gene therapy involves withdrawing the patient’s own bone-marrow stem cells and using a viral vector to add a normal copy of the beta-globulin gene to the sickle stem cells. The modified stem cells are then reinfused into the patient, who then produces normal, disc-shaped red blood cells.
Over the past 22 years of research at the NIH, the Tisdale lab has been optimizing engineered viral vectors as a way to deliver the correctly encoded beta-globin gene. One of his latest iterations of viral vectors, when tested in animal models, was up to 10 times as effective as conventional vectors. With clinical trials underway, Tisdale’s team is working toward substantiating gene therapies as a viable method for stem-cell transplantations, even in the case of severe SCD (Nat Commun 10:Article number 4479, 2019). Thanks to Tisdale’s gene-therapy approach, so far several people—in a multicenter clinical trial that will have an estimated 50 participants—have been apparently cured of SCD. The trial is expected to be completed in 2022.
But that cure is not widely available. More than 20 million people worldwide have SCD, the majority in sub-Saharan Africa and India. “Ultimately, we need a strategy that is exportable to [the] part[s] of the world where the disease is most prevalent,” said Tisdale. Given the need for a blood bank and extensive infrastructure to support stem-cell transplants, there is much room to fine-tune and advance treatments. “We need simpler, less toxic ways to get the therapy to work,” he said. With recent advancement with CRISPR-Cas9 gene editing, the prospect, while far off, now seem possible, according to Tisdale.
Backline Approach to Mentorship
As a bass guitarist, the backline is where Tisdale is comfortable, placed toward the back of the stage, helping those around him shine while holding the song together with a steady rhythm. He takes a similar approach to mentoring the next generation of sickle-cell researchers. Tisdale has overseen the training of many fellows and students. “What I like to do is to find people who are internally motivated, who have the passion to do something to help humanity,” he said. “[I] just get out of their way.”
As mentees become collaborators, they are becoming part of a well-developed NIH consortium—that Tisdale helped establish—dedicated to addressing the challenges still facing sickle-cell treatment. Together, Tisdale and his colleagues are working toward expanding treatment to everyone with SCD.
Former mentee Courtney Fitzhugh (now a Lasker Clinical Research Scholar in NHLBI) is independently implementing clinical trials involving half-match bone-marrow donors—such as a parent, child, or half-matched siblings—as a viable method of allogeneic stem-cell transplantation to treat SCD. “For the first time we have this dilemma of three potentially curative therapies to choose from: 1) full match, 2) half match, [and] 3) autologous gene therapies,” said Tisdale.
Restoring the Seemingly Unfixable
To Tisdale, “The most satisfying thing is to go to a clinic, to see a patient through follow-up who has been through an experimental protocol and see their life totally changed.” Perhaps what music and medicine have in common is the ability to heal people and ease their pain through either a soothing rhythm or a curative therapy.
Tisdale enjoys restoring other seemingly unfixable things. At the end of the interview, he showed this writer a video of the product of one of his hobbies—restoring pianos from the late 1700s and early 1800s. The deep buzz of a recently restored 1786 Longman and Broderip piano reverberated across the audio. The brass and steel strings effortlessly vibrated against the soundboard, a tribute to the care put in place by the person who sought to restore the seemingly unfixable piano, John Tisdale.
John Tisdale gave a Philip S. Chen Jr. Distinguished Lecture on Innovation and Technology Transfer, entitled “The Long and Winding Road toward Molecular Cures of the First Molecular Disease,” on November 8, 2019. To watch a videocast, go to https://videocast.nih.gov/watch=35155. He and his sickle-cell work were also featured in the Discovery Channel’s First in Human documentary (aired in August 2017) that was filmed at the NIH Clinical Center: https://www.youtube.com/watch?v=tnF4UYKzVbQ. In addition, Tisdale’s work was featured on an episode of 60 Minutes (aired on March 10, 2019): https://www.cbsnews.com/news/could-gene-therapy-cure-sickle-cell-anemia-60-minutes/.
JOHN TISDALE, M.D.
Senior Investigator and Chief, Cellular and Molecular Therapeutics Branch, National Heart, Lung, and Blood Institute

PHOTO BY: CHIA-CHI CHARLIE CHANG, OD
John Tisdale
Education: M.D. from Medical University of South Carolina (Charleston, South Carolina)
Training: Residency in internal medicine, Vanderbilt University School of Medicine (Nashville); chief resident, Veterans Affairs Medical Center (Nashville)
Came to NIH: In 1994 as a hematology fellow in NHLBI; held various positions in the National Institute of Diabetes and Digestive and Kidney Diseases (1998–2007); was tenured in 2006; moved to NHLBI in 2007
Website: https://irp.nih.gov/pi/john-tisdale
This page was last updated on Thursday, March 24, 2022
