Research Briefs
NIAID: EXPERIMENTAL RESPIRATORY SYNCYTIAL VIRUS VACCINE PROMPTS ANTIBODY SURGE
CREDIT: NIAID
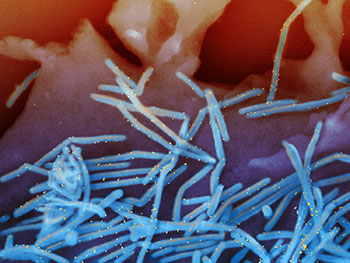
Scanning electron micrograph of human respiratory syncytial virus (RSV) virions (colorized blue) and labeled with anti-RSV F protein/gold antibodies (colorized yellow) shedding from the surface of human lung epithelial cells.
A novel experimental vaccine against respiratory syncytial virus (RSV), a leading cause of severe respiratory illness in the very young and the old, has shown early promise in a phase 1 clinical trial. The candidate vaccine, DS-Cav1, was engineered and developed by researchers at NIAID’s Vaccine Research Center (VRC), who were guided by their atomic-level understanding of the shape of an RSV protein. An interim analysis of study data showed that one dose of the investigational vaccine prompted large increases in RSV-neutralizing antibodies that were sustained for several months.
Previous candidate RSV vaccines made with traditional techniques, such as by inactivating the whole virus or by making subunit vaccines without attention to protein conformation, failed. Structural biology techniques now permit researchers to visualize in minute detail areas of viral proteins, called epitopes, recognized by the immune system. Over several years, the VRC researchers used structural information to develop a candidate RSV vaccine that could stimulate neutralizing antibodies. In 2013 they tested several versions as a vaccine in both mice and nonhuman primates. These protein variants elicited high concentrations of neutralizing antibodies and protected the animals against RSV infection.
The most promising, DS-Cav1, was selected for clinical evaluation and subsequently manufactured for clinical testing by the VRC. “Four weeks after immunization, the [DS-Cav1] vaccine [had] elicited substantially more high-quality antibody titers than those typically generated using earlier RSV immunogens,” according to the paper. “The findings provide a proof of concept for how structural biology can contribute to precision-vaccine design.” (NIH authors: M.C. Crank plus 30-some authors including B.S. Graham and former postdoc Jason McLellan, who is now at the University of Texas at Austin, Science 365:505–509, 2019; DOI:10.1126/science.aav9033)
NIDDK, NCI, NIAID, NIAMS: “WILDLING” MICE COULD HELP TRANSLATE RESULTS IN ANIMAL MODELS TO RESULTS IN HUMANS
CREDIT: Reprinted with permission from Rosshart et al., Science 365:eaaw4361 (2019)
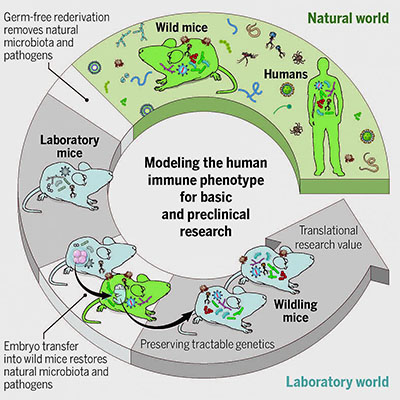
In the current study, they transplanted embryos of the most commonly used strain of laboratory mice for immune-system research into female wild mice, who then gave birth to and raised wildlings. The researchers compared the microbiota of the wildlings, wild mice, and lab mice. They found that the wildlings acquired the microbes and pathogens of wild mice and closely resembled wild mice in their bacterial microbes present at the gut, skin, and vagina, as well as in the number and kinds of fungi and viruses present.NIH researchers and others, led by scientists from NIDDK, developed a new mouse model that could improve the translation of research in mice into advances in human health. The mouse model, which the scientists called “wildling,” acquired the microbes and pathogens of wild mice while maintaining the laboratory mice’s genetics, which make them more useful for research. In two preclinical studies, wildlings mirrored human immune responses, whereas lab mice failed to do so. The researchers previously showed that transferring wild-mice gut microbiota into lab mice helped the mice survive an otherwise-lethal flu virus infection and fight colorectal cancer. (Cell 171:1015–1028, 2017; DOI:10.1016/j.cell.2017.09.016)
The researchers also tested the stability and resilience of the wildlings’ microbiota and found the microbiota was stable across five generations and resilient to environmental challenges. The authors suggest that the stability and resilience of wildlings, if the model is used widely, could improve the validity and reproducibility of biomedical studies.
Finally, the researchers tested how well the wildlings could predict human immune responses. To do so, they drew from two studies in which drugs used to target immune responses were successful in treating lab mice in preclinical trials but then failed to have therapeutic effects in humans. In the current study, the researchers treated wildlings and lab mice with the same drugs. The wildlings, but not the lab mice, mimicked the human responses seen in clinical trials.
“Such models may enhance the validity and reproducibility of biomedical studies among research institutes, facilitate the discovery of disease mechanisms and treatments that cannot be studied in regular laboratory mice, and increase the translatability of immunological results to humans,” the authors wrote. (NIH authors: 19 including lead author S.P. Rosshart and senior author Barbara Rehermann, Science 365:eaaw4361, 2019; DOI:10.1126/science.aaw4361)
NIAID (ROCKY MOUNTAIN LABORATORIES): EXPERIMENTAL TREATMENT SLOWS PRION DISEASE, EXTENDS LIFE OF MICE
CREDIT: NIAID
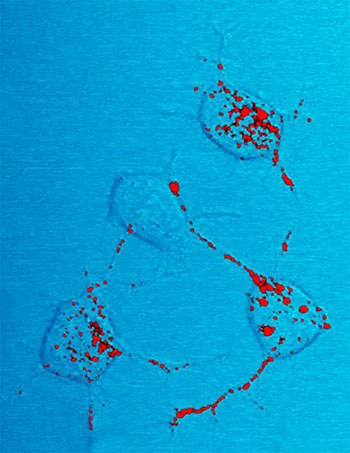
Prion protein, shown in red, can become infectious and cause neurodegenerative disease. Here four nerve cells in a mouse illustrate how infectious prion protein moves within cells along neurites—wire-like connections the nerve cells use for communicating with adjacent cells.
Scientists at NIAID’s Rocky Mountain Laboratories (Hamilton, Montana)—in collaboration with researchers at the Broad Institute (Cambridge, Massachusetts) and other organizations—used an experimental treatment in laboratory mice to slow the progression of scrapie, a degenerative central nervous system disease caused by prions, and greatly extended the rodents’ lives. The scientists used antisense oligonucleotides (ASOs), synthetic compounds that inhibit the formation of specific proteins.
Prion diseases occur when normally harmless prion protein molecules become abnormal and gather in clusters and filaments in the body, including in the brain. The diseases are thought to alway be fatal. Scrapie, which affects sheep and goats and can be adapted to infect rodents, is closely related to human prion diseases such as Creutzfeldt-Jakob disease, which is currently untreatable.
In the studies, the scientists injected ASOs into the spinal fluid of mice already infected with scrapie or that were challenged with scrapie proteins within weeks of the injection. The work “provides a particularly clear demonstration of the potential for ASOs to effectively treat neurodegenerative disorders by lowering a target protein in the brain,” the authors wrote. The researchers plan to expand their scrapie ASO studies to human prion diseases. Other researchers have seen promising initial results in humans with ASOs directed against Alzheimer disease, amyotrophic lateral sclerosis, and Huntington disease. (NIH authors: G.J. Raymond, B. Race, L.D. Raymond, K. Williams, L.L. Lubke, A. Kraus, E.V. Minikel, B. Caughey, and S.M. Vallabh, JCI Insight 4:e131175, 2019; DOI:10.1172/jci.insight.131175)
NIEHS: CELLULAR TRAFFIC COP DIRECTS DNA TRANSCRIPTION

CREDIT: MPW, CAN STOCK PHOTO
Nuclear transcription factor Y acts as a traffic cop, directing RNA polymerase II traffic to the correct transcription start site (TSS) while blocking other TSSs.
Faithful DNA transcription initiation is critical for accurate gene expression, yet the mechanisms underlying specific transcription start site (TSS) selection in mammals remain unclear. NIEHS researchers showed that the histone-fold domain protein nuclear transcription factor Y (NF-Y), a ubiquitously expressed transcription factor, controls the fidelity of transcription initiation at gene promoters in mouse embryonic stem cells. They reported that NF-Y directs RNA polymerase II (Pol II) so that it begins transcription at the right TSS.
The researchers found that, like a cop directing traffic away from a closed lane, NF-Y funnels the Pol II transcriptional machinery to the correct TSS while physically blocking other TSSs. The study showed that NF-Y defines where transcription starts by how it organizes the chromatin structure of the promoter. By acting as an enforcer to ensure the correct TSS is used, NF-Y protects against any negative consequences of initiating transcription from the wrong place. (NIH authors: A.J. Oldfield, T. Henriques, D. Kumar, A.B. Burkholder, S. Cinghu, B.D. Bennett, P. Yang, B.S. Scruggs, C.A. Lavender, K. Adelman, and R. Jothi, Nat Commun 10:Article number 3072, 2019; DOI:10.1038/s41467-019-10905-7)
NIDDK: THE MOLECULAR MECHANISMS BEHIND INSULIN SECRETION
CREDIT: JURGEN WESS, NIDDK
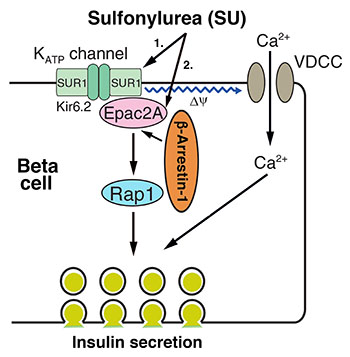
NIDDK scientists discovered a molecular mechanism for how sulfonylureas (SUs), a group of antidiabetic drugs, stimulate the beta cells in the pancreas to release insulin. Shown: The molecular mechanism of SU-induced beta-arrestin–1–dependent insulin secretion.
NIDDK scientists have discovered a novel molecular mechanism that is key to helping a group of antidiabetic drugs called sulfonylureas (SUs) stimulate insulin secretion. SUs, which have been widely used to treat type 2 diabetes for more than 50 years, work by increasing insulin release from the beta cells in the pancreas.
The researchers found that two widely used antidiabetic SUs—glibenclamide and tolbutamide—weren’t effective in mice that lacked a protein called beta-arrestin 1 (Barr1) in insulin-secreting pancreatic beta cells. Lack of Barr1, however, did not affect stimulation of insulin secretion by glucose or other agents known to promote insulin release. Using state-of-the-art molecular biology techniques, the researchers noticed that SUs enable Barr1 to bind with a protein called exchange protein directly activated by cyclic AMP 2 (Epac2); the resulting complex enhances the activity of a protein called Ras-related protein 1, which eventually triggers enhanced insulin secretion.
“Our study suggests that therapeutic strategies that promote beta-cell Barr1 signaling may prove beneficial for the development of successful antidiabetic drugs,” said Luiz F. Barella, lead author of the study and a postdoctoral fellow in NIDDK’s Laboratory of Bioorganic Chemistry. (NIH authors: L.F. Barella, M. Rossi, L. Zhu, Y. Cui, and J. Wess, J Clin Invest, 2019; DOI:10.1172/JCI126309)
NIDDK, NHLBI, NICHD: MICE LACKING A PROTEIN IN ADIPOCYTES SHOWED PRONOUNCED METABOLIC IMPROVEMENTS
Research led by scientists at NIDDK found that mice lacking the beta-arrestin-2 (Barr2) protein in adipocytes (cells specialized for the storage of fat) showed decreased body weight, fat mass, and blood-glucose concentrations, and increased glucose and insulin tolerance when maintained on a high-fat diet. The researchers also found that these metabolic improvements were associated with the browning of white fat and increased energy expenditure.
This research was the first to show the Barr2 protein as a critical regulator of whole-body glucose and energy homeostasis in adipocytes. Also playing a role in this research are G proteins, a family of proteins that transmit signals to a cell’s interior. Data support the novel concept that G-protein-biased agonists of beta-3-adrenergic receptors, located mainly in adipose tissue and involved in promoting lipolysis, that do not promote interactions with Barr2 may prove clinically useful for the treatment of obesity and related metabolic disorders. (NIH authors: S.P. Pydi, S. Jain, W. Tung, Y. Cui, L. Zhu, W. Sakamoto, S. Jain, B.S. Abel, M.C. Skarulis, J. Liu, T. Huynh, K. Pacak, O. Gavrilova, T. Finkel, and J. Wess, Nat Commun 10:Article number 2936, 2019; DOI:10.1038/s41467-019-11003-4)
NIDA: IN ANIMAL STUDY, FEMALE REPRODUCTIVE CYCLE AFFECTS COCAINE CRAVING
Scientists are interested in how drug-seeking behavior increases during abstinence (a.k.a. “incubation of craving”). NIDA researchers wanted to determine whether the incubation of craving would be different in male and female rats that were exposed to cocaine and whether the effect is stronger after binge cocaine intake, compared with continuous use.
In this study, the rats self-administered cocaine either continuously or intermittently (modeling binge-use patterns seen in people) over an 8-hour period. This administration schedule was followed for 12 days. Then, after either 2 or 29 days without access to cocaine, the animals were once again placed in the same environment where they previously self-administered cocaine, and the researchers measured the response for cocaine-associated cues.
In the rats, cocaine seeking was higher after intermittent drug access than continuous access, suggesting that binge use might prompt stronger cravings. Interestingly, cocaine seeking was higher in female rats than in male rats in both models. In fact, in female rats, incubation of craving after either intermittent or continuous drug access was significantly higher during estrus, the sexually receptive period in their reproductive cycle. The scientists note that a question for future research would be to examine whether cocaine craving in female humans is modulated by the menstrual cycle. (NIH authors: C. Nicolas, T.I. Russell, A.F. Pierce, S. Maldera, Z.-B. You, Y. Shaham, and S. Ikemoto, Biol Psychiatry, 85:915–924, 2019; DOI:10.1016/j.biopsych.2019.01.015)
NICHD: AIR POLLUTION LINKED TO INCREASE IN NEWBORN INTENSIVE CARE UNIT ADMISSIONS
Infants born to women exposed to high amounts of air pollution in the week before delivery are more likely to be admitted to a newborn intensive care unit (NICU), suggests an analysis by NICHD researchers. Depending on the type of pollution, chances for NICU admission increased from about 4% to as much as 147%, compared with rates for infants whose mothers did not encounter high amounts of air pollution during the week before delivery. If the findings are confirmed, the study suggest that pregnant women may want to consider limiting their time outdoors when air-quality advisories indicate unhealthy conditions.
The researchers analyzed data from the Consortium on Safe Labor, which compiled information on more than 223,000 births at 12 clinical sites in the United States from 2002 to 2008. They linked records from more than 27,000 NICU admissions to data modified from the Community Multiscale Air Quality Modeling System, which estimates environmental pollution concentrations in the United States.
Chances of NICU admission increased significantly when the women were exposed to traffic-related pollutants on the day before and the day of delivery compared with the week before delivery. The researchers do not know why exposure to air pollution might increase the chances for NICU admission. They theorize, however, that pollutants increase inflammation, leading to impaired blood-vessel growth, particularly in the placenta, which supplies oxygen and nutrients to the developing fetus. If their results are confirmed by other studies, limiting pregnant women’s exposure to high amounts of air pollutants may provide a way to reduce NICU admissions. (NIH authors: I. Seeni, A. Williams, C. Nobles, Z. Chen, and P. Mendola, Ann of Epidemiol, 2019; DOI:10.1016/j.annepidem.2019.07.008)
NCBI: THE ROLE OF REPETITIVE DNA AND PROTEIN SEQUENCES IN TUMOR EVOLUTION
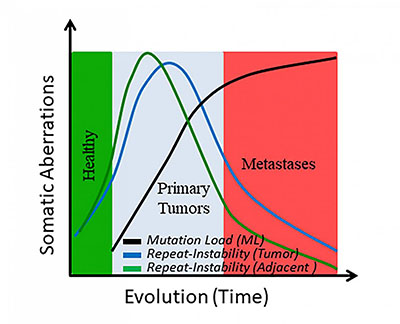
CREDIT: EREZ PERSI, NCBI
Graph of somatic changes over time.
A team of researchers from NCBI (in the National Library of Medicine) and collaborating academic research institutions developed a method to measure a type of gene mutation involved in the evolution of cancer. This type of mutation, called “repeat instability,” may be useful in early cancer diagnosis.
The researchers developed a computational methodology to quantify variations in the repeat content of gene and protein sequences. They analyzed genetic-sequence data from 325 patients with a variety of cancers, including breast, prostate, bladder, and lung cancers, as well as individual patients with metastases. Using computational-biology techniques, the researchers compared the sequences from the cancer tissue with those from healthy tissue adjacent to the cancer site and to those in blood, which served as the control.
The study showed that repetitive sequences, which are hotspots of DNA evolution, emerge early in tumor evolution but fade away in later phases, particularly during the transition to metastatic states. “The study found that noncancerous tissue adjacent to tumors had patterns of repetitive sequences that were similar to those detected in tumors,” said Erez Persi, lead author on the paper. “This fact, and the reduction in repeat sequences seen once the cancers metastasized, suggests the potential for using repeat sequences in the early diagnosis of cancer.” (NIH authors: E. Persi, Y.I. Wolf, and E.V. Koonin, Proc Nat Acad Sci U S A 116:16987–16996; 2019; DOI:10.1073/pnas.1908790116)
This page was last updated on Thursday, March 31, 2022
