New Methods
A Simpler Way to Make a Conjugate Vaccine for Cholera
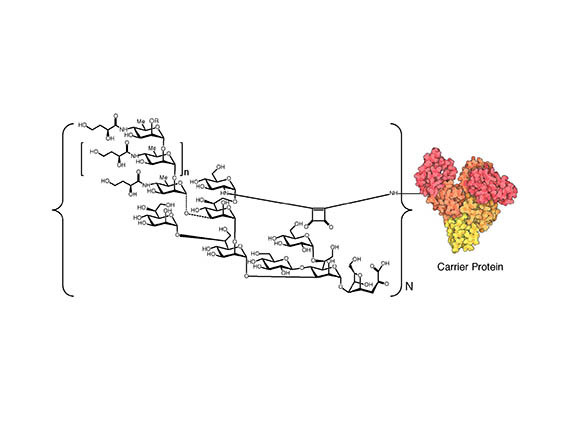
CREDIT: PENG XU AND PAVOL KOVAC, NIDDK
Diagram showing the general molecular structure of V.cholerae O1/protein conjugates. To the left is the structural formula of the polysaccharide. (O=oxygen; OH=hydroxyl group; NH=a secondary amino group; Me=methyl group; OR=different substitute groups; the “n” outside the small brackets means there are a different number of repeating monosaccharide units; the “N” outside the bracket means there are a different number of copies of the polysaccharide attached on the surface of the protein). To the right is depiction of the carrier protein.
Researchers at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) have developed an easier, less expensive way to produce a conjugate cholera vaccine against Vibrio cholerae serogroup O1, the globally dominant cause of cholera.
“The invention has been [recently] patented and is also being applied to making conjugate vaccines for other infectious diseases,” said NIDDK scientist emeritus Pavol “Paul” Kovac, who retired earlier this year. He and NIDDK Staff Scientist Peng Xu collaborated with researchers from Harvard Medical School (Boston) and the International Centre for Diarrhoeal Disease Research (Dhaka, Bangladesh) in developing the new method.
Cholera is a severe diarrheal illness caused by the ingestion of food or water that has been contaminated by V. cholerae bacteria—usually by fecal matter from infected people. Cholera epidemics have occurred all over the world, especially in areas where there is violent conflict, drought, famine, and displaced populations. In 2017, according to the World Health Organization, there were 1.2 million reported cases in 34 countries—including Yemen, the Democratic Republic of the Congo, and Haiti—with 5,654 deaths; in the United States there were 11 cases and no deaths.
Injectable cholera vaccines were first developed in labs in the late 1800s; oral versions of the vaccines were introduced in the 1980s. There are several types of oral cholera vaccines today, but they provide only 65% to 85% protection for six months to five years, depending on the vaccine.
Bacterial carbohydrate antigens—such as O-antigens from V. cholerae—do not elicit a strong-enough immune response. A conjugate vaccine, however, triggers a stronger immune response because it chemically combines a weak antigen (usually a polysaccharide) to a strong protein antigen.
Conjugate vaccines were first tested in 1927 against Streptococcus pneumoniae in rabbits. The first conjugate vaccine to be used in people was the Haemophilus influenzae type b conjugate vaccine (developed by NIH scientists Rachel Schneerson and John Robbins), which protects against meningitis. It became available in 1987 and was soon included in the infant immunization schedule in the United States. The first conjugate vaccines against cholera were developed and tested in clinical trials in the 1990s.
The trouble with the existing conjugate cholera vaccines is that making them is a complex, multistep process that involves adding chemical linkers to enable the two components to be joined to each other. Then the resulting linker-equipped intermediates have to be purified, which adds to the cost.
Kovac and Xu’s method, however, is more direct and involves fewer steps. The invention is based on the ability of squaric acid (so-named because its four-carbon atom ring resembles a square) chemistry to join the polysaccharide directly to the protein component. The purification of the antigen intermediate and the resulting glycoconjugate vaccine is also a simpler process than the one used conventionally.
The approach “produces conjugates that are fully and specifically recognized by immune responses in humans,” the authors wrote in their 2011 paper describing the method. (Bioconjugate Chem 22:2179–2185, 2011; DOI:10.1021/bc2001984)
“Nobody ever tried to conjugate bacterial polysaccharides to proteins to make vaccines without using linkers,” said Kovac. “We’ve developed a simpler, [more] efficient method.”
He explained that the squaric acid chemistry method was first developed in Germany in 1992 by L.F. Tietze. (Bioconjugate Chem 2:148–153, 1991; DOI:10.1021/bc00009a003).
“The method was never tried to conjugate bacterial polysaccharides,” said Kovac. “We improved it and optimized reaction conditions.”
An outside biotech company has verified that the chemistry works and that the process is scalable to an industrial level. The vaccine has not yet gone into clinical trials.
Some of the background material used in this article is from Harris, Jason B., “Cholera: Immunity and prospects in vaccine development,” J Infect Dis (Suppl 3):S141–S146, 2018; DOI:10.1093/infdis/jiy414.
This page was last updated on Thursday, March 31, 2022
