Fostering Stem Cell Research at NIH
2019 NIH Stem Cell Symposium
Stem-cell research being performed by NIH intramural and extramural scientists was highlighted at the 2019 NIH Stem Cell Symposium, held on May 13, 2019, in Lipsett Amphitheater (Building 10). The event was organized by stalwarts in stem-cell research: Barbara Mallon, the technical director of the NIH Stem Cell Unit; Pamela Gehron Robey, a senior investigator in the National Institute of Dental and Craniofacial Research and acting scientific director of the NIH Stem Cell Unit; Ilyas Singeç, the director of the Stem Cell Translational Laboratory at the National Center for Advancing Translational Sciences; and Jizhong Zou, the director of the Induced Pluripotent Stem Cell (iPSC) Core Facility at the National Heart, Lung, and Blood Institute (NHLBI).
The symposium featured talks by intramural and extramural scientists as well as poster sessions. Following are highlights of the intramural presentations.
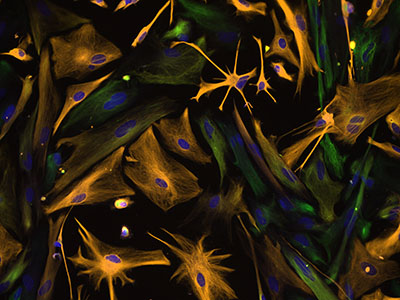
CREDIT: CAROL IBE, EUGENE MAJOR, NINDS
Directed Differentiation of Multipotential Human Neural Progenitor Cells to Astrocytes: Human neural progenitor cells were isolated under selective culture conditions from the developing human brain and directed through lineage differentiation to astrocytes. After three weeks in astrocyte-selective medium, cells were fixed and stained with antibodies to intermediate-filament proteins that characterize the cells as either astrocytes (orange) or neural progenitor cells (green); nuclei are stained blue. Microscopic examination over the course of differentiation showed loss of progenitor cells as the cell population increasingly became astrocytes. This photomicrograph was taken on Axiovert 200M Zeiss inverted microscope at 200x magnification.
Development and Biology of Adult Stem Cells
Stadtman Investigator Ramiro Iglesias-Bartolome (National Cancer Institute, NCI) is elucidating the signaling mechanisms that control and drive tissue-specific stem-cell self-renewal and differentiation and their connections to tumor initiation and growth. He described his team’s work exploring the regulation of epithelial stem-cell differentiation and proliferation by heterotrimeric guanine nucleotide-binding (G) proteins.
G-protein-coupled receptors (GPCRs) make up the largest family of cell-surface molecules involved in cell signal transduction, physiological processes, and pathological conditions. Recently his team demonstrated in mice that conditional epidermal deletion of the gene coding for the stimulatory G-protein alpha heteromeric subunit (Gnas) or the inactivation of protein kinase A are sufficient to cause an aberrant expansion of basal progenitor keratinocytes in the skin, resulting in basal-cell carcinoma. His team is further investigating the role of coupled GPCRs in the regulation of stem-cell differentiation and proliferation in the skin.
David Bodine (National Human Genome Research Institute) focuses on hematopoiesis (formation of blood cells) and erythropoiesis (the regenerative process in which undifferentiated hematopoietic cells differentiate into red blood cells). When these processes go awry, a variety of disorders ranging from hematologic malignancy to anemia can result. At the symposium, he described his lab’s work on single-cell analysis of hematopoiesis. In human hematopoiesis, the bone-marrow cells—megakaryocytes and erythroid cells—differentiate from a shared precursor, the megakaryocyte-erythroid progenitor (MEP). His team recently reported that the analysis revealed that human MEP contains three populations of cells.
In another project, Bodine’s lab is comparing the epigenetic profiles of normal and Diamond-Blackfan anemia (DBA) patient cells to determine whether specific genes and pathways are altered. DBA is a rare inherited bone-marrow-failure syndrome. Bodine envisages that the results of these studies may lead to potential new therapies for DBA and other anemias.
Development and Biology of Pluripotent Stem Cells
To achieve precision use of desired human pluripotent stem cells (hPSCs) for medical and pharmaceutical applications, it is essential to have a thorough understanding of all fundamental properties of these starting cell sources. One of the most important properties is related to the naive pluripotent state that is primarily established in mouse embryonic stem cells.
Staff Scientist Kevin Chen (National Institute of Neurological Disorders and Stroke) talked about how his current research on characterizing pluripotent stem cells (PSCs) and adult stem cells aims to identify optimal signaling cascades that precisely control diverse pluripotent and differentiated states in vivo and in vitro. He is unraveling genetic and biochemical pathways that regulate stem-cell genome stability, growth rates, and differentiation trajectories in various stem-cell culture and differentiation platforms. He described how PSCs are used to model various neuronal and vascular diseases in vitro for drug discovery and to optimize neuroectodermal and mesoendodermal differentiation in cell-culture platforms for regenerative medicine.
He also talked about oligodendrocyte progenitor cells (OPCs), which are endogenous stem cells in the central nervous system that serve as the predominant source of myelinating oligodendrocytes. The loss or dysfunction of OPCs can lead to significant motor and cognitive disability in patients due to myelination defects.
To better understand rare and inherited disorders, Senior Investigator Manfred Boehm (NHLBI) and his team developed a patient-specific induced PSC (iPSC) platform that includes cardiovascular lineage differentiation as well as iPSC-related cell-differentiation platforms to generate endothelial cells, vascular smooth-muscle cells, and mesenchymal and hematopoietic cell lineages. His team is developing complex models for cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (the most common form of hereditary stroke disorder) and other conditions by expanding iPSC technology to multicellular human organoid xenotransplantation systems.
New Technologies and Models
Clinical translation of hPSCs requires advanced strategies that ensure safe and robust long-term growth and functional differentiation. Pluripotent cells are capable of extensive self-renewal yet remain highly sensitive to environmental perturbations in vitro, posing considerable challenges to their therapeutic use. Ilyas Singeç (National Center for Advancing Translational Sciences) and his team deployed innovative high-throughput screening strategies to identify a small-molecule cocktail that dramatically improves viability of hPSCs and their differential progeny. The team discovered that the combination of four factors, termed “CEPT,” enhances cell survival of genetically stable hPSCs by simultaneously blocking several cellular-stress mechanisms. According to Singeç, CEPT represents a broadly applicable chemical platform for comprehensive cytoprotection (a process by which chemical compounds provide protection to cells against harmful agents), providing a novel strategy for cell survival and efficient use of hPSCs. Human PSCs have the potential to generate all cell types of a human body under 2-D culture conditions or form organoids under 3-D cell-culture conditions. Cerebral organoid cultures from human iPSCs have recently been developed to recapitulate the cytoarchitecture of the developing brain. These human iPSC–based model systems offer unique advantages in understanding molecular and cellular mechanisms governing embryonic development and in modeling neurodevelopmental disorders, such as brain malformation and neuropsychiatric disorders.
Using the first iPSC line from a hibernating mammal (13-lined ground squirrel, Ictidomys tridecemlineatus), Jinxing Ou (National Eye Institute) uncovered cellular pathways critical for cold tolerance. Comparison between human and ground squirrel iPSC-derived neurons revealed differential mitochondrial and protein quality-control responses to cold. Thus, ground squirrel iPSCs offer a unique platform for bringing cold-adaptive strategies from hibernators to humans in clinical applications.
Translational Stem-Cell Biology
T cells are potentially curative for patients with metastatic cancer, but many patients with cancer have T cells that are terminally differentiated, a condition associated with treatment failure.
Nicholas P. Restifo (NCI)—who is developing immunotherapeutic treatments for patients with metastatic cancer and focusing on every aspect of understanding the interactions of T cells with tumors—observed that less-differentiated T cells have a greater capacity to proliferate, persist, and destroy large cancer deposits. Generally, T cells can be generated from iPSC in vitro by co-culturing them from OP9 stromal cells. Restifo’s lab demonstrated that tumor-specific T cells can be generated from iPSC using 3-D thymic-organ tissue. Further, the lab will be using these cells to treat patients with metastatic cancer; iPSC-derived T cells have a potentially unlimited capacity for proliferation, engraftment, and antitumor activity.
Cardiac-cell therapies show promise as a strategy for myocardial repair, but one of the challenges is understanding how cell grafts behave long-term. Scientists, therefore, need technologies that enable longitudinal monitoring of transplanted cells. So Gun Hong (NHLBI), described how she and Cynthia E. Dunbar (NHLBI) developed sodium-iodide symporter (NIS)–based in vivo imaging to detect and track rhesus monkey iPSC (RhiPSC)–derived cardiomyocytes in a murine model of myocardial infarction. The rhesus NIS gene was incorporated into RhiPSCs via CRISPR/Cas9 gene editing. The cardiomyocytes derived from NIS-RhiPSCs exhibited normal morphological and electrophysiological characteristics under physiologic conditions. This NIS-based molecular-imaging platform, with optimal safety and sensitivity characteristics, is primed for translation into large-animal preclinical and clinical trials.
To view a videocast of the symposium, which was held on May 13, 2019, go to https://videocast.nih.gov/launch.asp?27536 (NIH only). To read more about research in the Stem Cell Unit, go to https://stemcells.nih.gov/.
This page was last updated on Monday, April 4, 2022
