2018 Research Festival: Plenary Sessions
- Plenary I: The Institute Directors
- Plenary II: Unity Is Strength: A Journey into Disease Discovery and Treatment
- Plenary III: Devising Therapeutic Strategies to Combat Allergies and Flu
Plenary I: The Institute Directors
“The Most Important Nine Months of Your Life”
Diana Bianchi (Director, Eunice Kennedy Shriver National Institute of Child Health and Human Development)

CREDIT: MARLEEN VAN DEN NEST
“What happens during the time you are in the womb influences your lifelong health,” said Bianchi. She highlighted the collaborative NICHD Human Placenta Project, which aims to understand the role of the placenta—in the mother and baby—in health and disease both before and after birth. “Pregnancy is a stress test for risk of later diseases such as myocardial infarctions, stroke, diabetes, [and, later,] vascular dementia in the mother,” said Bianchi.
CREDIT: NICHD
Model of a placenta.
There are fetal origins for many diseases—such as cardiovascular disease, hypertension, cancer, obesity, allergies, and even mental illness—that stem from problems with the placenta. Birth weight outside the normal ranges is a huge risk factor for all these conditions, yet internists rarely ask adults about their birth weight. NICHD’s supported research includes an extramural project that uses microvascular imaging to visualize fetal and maternal circulation to noninvasively assess the health of the placenta; an intramural-extramural collaboration to develop a wireless device that continuously monitors oxygenation of the placenta; intramural research on genomics, placenta-omics, and ways to reduce invasive fetal tests. Bianchi also described her own research in her lab at the National Human Genome Research Institute where she is studying prenatal genomics to inform novel therapies for genetic conditions.
“Mental Health Research: Priorities and Progress”
Joshua Gordon (National Institute of Mental Health)

CREDIT: MARLEEN VAN DEN NEST
“Death by suicide…has been increasing continually for the past almost 20 years…particularly in children and adolescents,” said Gordon, who highlighted suicide prevention, mapping neural circuits, and computational psychiatry as three priority research areas for NIMH. He described an extramural study in which investigators showed that if all people who come to the emergency room were asked if they were thinking about suicide, “you identify about twice as many people who are at risk for suicide [than] if you only asked the people that you have some clinical suspicion about.” An intervention consisting of three follow-up phone calls over the following year led to a 30 percent decrease in suicide attempts. NIMH intramural researchers have developed a suicide-screening tool called the Ask Suicide-screening Questions (ASQ) that “really works in kids,” said Gordon.
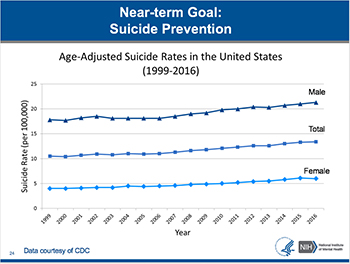
CREDIT: CDC
One of NIMH’s priorities is to prevent suicide, which is the 10th leading cause of death overall, and has not decreased since 1999. While the suicide rate for males is approximately 4 times higher than females, the rates for both males and females have increased over the past 17 years.
In other projects, researchers are using optogenetics and other neurocircuitry technologies to map neural circuits gone awry in anxiety disorders in mice; and computational methods to build accurate models of how neurocircuits work to understand and predict psychiatric problems. His own research (his lab is in the National Institute of Neurological Disorders and Stroke) focuses on neural activity and genetics in mouse models of psychiatric diseases such as schizophrenia, anxiety disorders, and depression.
“Advancing Health through Accelerating Information Access”
Patricia Flatley Brennan (Director, National Library of Medicine)

CREDIT: MARLEEN VAN DEN NEST
“The National Library of Medicine is almost 180 years old, and we’ve been able to touch almost every single discovery of health information in the past 20 years,” said Brennan. She went on to describe four research programs that focus on bioinformatics resources: computational approaches to basic biology; computational approaches to improve public health; tools for better research; and discovery from health data.

CREDIT: NLM
Patricia Brennan described a tool that provides real-time flu surveillance.
Among the many projects she mentioned was using computational approaches to identify new proteins resulting from CRISPER-Cas editing, predict causes of mutations in cancer-driving genes, and quickly identify emerging foodborne pathogens; using data assimilation to help individuals forecast glucose levels after meals; developing better tools to interpret and synthesize research findings in the mass collections of journal articles; and using machine learning to detect social-media posts indicating prescription-medication abuse. Brennan, who is a registered nurse with a Ph.D. in industrial engineering, has a lab in the National Institute of Nursing Research where she uses virtual reality to predict how well patients will manage at home after being discharged from the hospital.
“Ending the HIV Epidemic”
Anthony Fauci (Director, National Institute of Allergy and Infectious Diseases)

CREDIT: MARLEEN VAN DEN NEST
Fauci, who has been studying HIV and AIDS since the disease emerged in the early 1980s, discussed advances in treatment and prevention of the AIDS pandemic. “The major breakthrough, which is truly historic, is in the arena of treatment,” said Fauci. In 1996, with the advent of the protease inhibitors, antiretroviral therapy (ART) could be used to treat people with AIDS and keep the virus below detectable levels in the blood indefinitely. “It was one of the most dramatic things I experienced in medicine,” said Fauci. “Individuals who were clearly on their way to dying were getting out of bed, going back to work, going back to their normal life.”
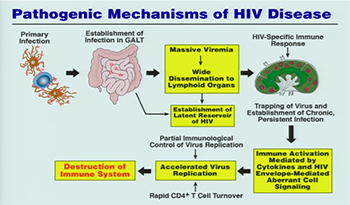
CREDIT: NIAID
Today, there are even better drugs. There are now 21 million people on ART and 10 million deaths were averted from 2000 to 2016. Prevention strategies have been “highly effective alone or in combination,” said Fauci. For example, research has shown that the one-pill-a-day pre-exposure prophylaxis (PrEP) regime is more than 95 percent effective in preventing the acquisition of HIV infection. Even better would be a long-term injectable dose of PrEP or, better still, an HIV vaccine. Researchers in NIAID’s Vaccine Research Center are working hard on developing such a vaccine.
“Science alone is not going to do it,” said Fauci. “It has to be scientific advances that are implemented in a very aggressive way. That is where political will, resources, and sustained commitment comes in. If we do that, I am really quite confident that within our lifetime…we will actually see the end of the HIV-AIDS pandemic.”
To watch a videocast of the “Plenary I: The IC Directors” session, which took place on Wednesday, September 12, 2018, go to https://videocast.nih.gov/launch.asp?25039.
Plenary II: Unity Is Strength: A Journey into Disease Discovery and Treatment
“Primary defects of immune function” and a “new lysosomal-storage disease” may sound like a strange combination of topics for a plenary session. But they do have something in common: Research advances in both areas have depended on successful collaborations that cross institute boundaries and are between basic scientists and clinicians. Jennifer Kanakry (NCI-CCR) and Luigi Notarangelo (NIAID) teamed up to talk about their collaboration on primary immunodeficiency diseases (PIDs); William Gahl (NHGRI) and Joseph Mindell (NINDS) told their story of how they identified and treated a new lysosomal-storage disease.
People with PIDs are at high risk for virus-associated lymphoproliferative disorders and cancers, immune dysregulation, autoimmunity, and life-threatening infections. Although PID can be treated with allogeneic bone-marrow transplants (in which a person receives hematopoietic stem cells from a donor), Kanakry explained that there are many complications. To reduce the likelihood of transplantation rejection, the researchers implemented a process known as conditioning ahead of time. The conditioning usually consists of chemotherapy and/or radiation and can have severe short- and long-term side effects including organ damage.
In collaboration with NIAID investigators, Kanakry led a clinical trial at NIH to test a less toxic conditioning strategy that contained no radiation and lower amounts of chemotherapy than typical approaches. She noted the trial outcomes were “remarkable,” resulting in 90 percent survival through day 180, low rates of transplant rejection and acute graft-versus-host disease, and no chronic graft-versus-host disease (an unwanted and often serious complication of transplantation). All patients were off post-transplant immunosuppressants by day 180.
Although the trial results were promising overall, improvements might be made if the disease mechanisms were better understood. Notarangelo focuses on PIDs caused by mutations of genes involved in variable-diversity-joining [V(D)J] genetic recombination, a process in which maturing T and B cells randomly assemble different gene segments, allowing the expression of a diverse array of T- and B-cell receptors, which in turn enable the body to recognize a wide range of potential pathogens.
Several types of PIDs, including Omenn syndrome, severe combined immunodeficiency, and other forms of combined immunodeficiency, are caused by defects in two proteins crucial to this process, recombination activating gene 1 (RAG1) and RAG2. Notarangelo’s group has used a variety of methods—including knockout mouse models and patient-derived induced pluripotent stem cells (iPSCs)—to determine how mutations of the RAG proteins contribute to disease.
His team discovered that normal function of the RAG proteins is critical to enable cross-talk between developing T cells and thymic epithelial cells. Through this cross-talk, self-reactive T cells are either eliminated in the thymus or are converted to T regulator (Treg) cells, whose function is to prevent autoimmunity. In patients with partial defects of RAG protein function, the generation and diversity of Treg cells are compromised, leading to autoimmune manifestations. Finally, by correcting the RAG gene defect in patient-derived iPSCs, Notarangelo’s team has been able to restore the capacity to generate T cells in a test tube. This ability to fix the RAG gene may have possible implications for the development of gene therapy for this disease.

CREDIT: LUKE FILDES—UNKNOWN, PUBLIC DOMAIN
Bill Gahl, the director of the Undiagnosed Diseases Program, opened his presentation with an image of this painting that depicts a physician who is trying to determine what his patient has. Gahl and Joseph Mindell told their story of how they identified and treated a new lysosomal-storage disease. (CREDIT: LUKE FILDES—UNKNOWN, PUBLIC DOMAIN, https://commons.wikimedia.org/w/index.php?curid=9004645)
The second collaboration focused on in this session was between the Undiagnosed Diseases Program (UDP) and researchers in NINDS. The result was the diagnosis of a new lysosomal storage disease. Gahl is the director of the UDP, which sees about 125 patients a year and has discovered and diagnosed several rare disorders. The UDP has received thousands of applications since opening in 2008, with about 10 percent receiving a full diagnosis and another 30 percent receiving a partial one. Gahl described two recent cases, beginning with an 18-month-old girl who suffered from failure to thrive, intestinal problems, and an unusual form of albinism (her skin and hair were affected, but not her eyes). Further investigations showed that she displayed a phenotype indicative of a lysosomal storage disorder, in which the lysosome (the cell’s salvage or recycling system) doesn’t function properly.
Diagnosing a new disorder from a single case is difficult, but the UDP subsequently learned of a second patient—a 14-month-old boy from Ghana—who had similar symptoms in addition to enlarged liver, spleen, and kidneys. The UDP discovered via genetic sequencing that the first child had a de novo mutation in CLCN7, a gene that encodes a chloride transporter that helps determine pH concentrations within the lysosome; the second child had exactly the same mutation.
Mindell took over the story and described his studies of CLCN7 in a different context. Lysosomes are known to be important in the breakdown of bone; loss-of-function mutations in CLCN7 cause osteopetrosis (characterized by dense but usually fragile bones). But interestingly, the two patients did not suffer from that. Gahl and Mindell discovered that the mutation in the UDP cases caused the lysosomes to become too acidic. They knew that chloroquine, a drug approved by FDA to treat malaria and amebic infections outside the intestines, could decrease acidity in lysosomes as a side effect. In fact, they demonstrated that—in cells cultured from patients—the drug could bring lysosomes’ pH to normal. So they recommended giving the two children chloroquine.
With treatment, the boy from Ghana has improved: His kidneys have decreased in size, and he can now roll over. The girl is being treated at the University of Colorado School of Medicine (Aurora, Colorado); the results haven’t been reported yet.
The presenters emphasized how collaborations can speed up the journey to great discoveries and, in the process, find new treatments that may improve the lives of many patients.
To watch a videocast of the “Plenary II: Immunodeficiency, Rare Diseases, Genetic Disorders, and Membrane Proteins” session, which took place on Thursday, September 13, 2018, go to https://videocast.nih.gov/launch.asp?25041.
Plenary III: Devising Therapeutic Strategies to Combat Allergies and Flu
Whether they research annoying allergies or killer flus, NIH scientists are intent on finding therapeutic strategies that may rid the world of these scourges. Four researchers from the National Institute of Allergy and Infectious Diseases (NIAID) shared their stories: Ian Myles and Pamela A. Guerrerio on food and skin allergies; and Jeffery K. Taubenberger and Audray K. Harris on influenza and flu vaccines.
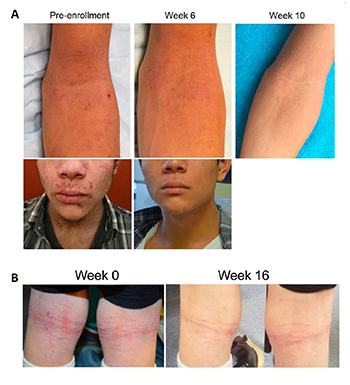
CREDIT: IAN MYLES, NIAID
Ian Myles and his team found that topical treatment with R. mucosa, a bacterium naturally present on the skin (and collected from healthy volunteers), relieves the severity of atopic dermatitis. Shown: Before and after photos of patients who took part in a clinical trial to test the effectiveness of R. mucosa treatment.
Myles spoke about a rosier future for atopic dermatitis (AD), also known as eczema. The underlying pathology of AD includes impaired skin-barrier function, susceptibility to Staphylococcus aureus skin infections, immune dysregulation, and cutaneous dysbiosis (a microbial imbalance on the skin). Myles and his team found that topical treatment with Roseomonas mucosa, a bacterium naturally present on the skin (and collected from healthy volunteers), relieves disease severity in mouse and cell-culture models as well as in humans.
Although scientists don’t know for sure what causes AD, studies indicate that the skin microbiome may play a role. The NIAID researchers conducted early-stage clinical trials at the NIH Clinical Center to test the safety of the R. mucosa treatment in 10 adult and five pediatric patients with AD. No complications or adverse effects occurred in either group, symptoms improved, and, in the children, the S. aureus population on their skin decreased.
The team also tested chemicals produced by R. mucosa and some found in certain skin products. They discovered that strains of R. mucosa in people with AD produced irritants (bacteria from healthy skin did not) and that some products may worsen AD and/or affect how well microbiome therapies work. His group is continuing its studies and expanding its clinical trials to determine whether bacteria transplants will be an effective way to treat AD in the future.
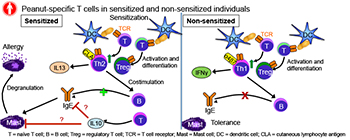
CREDIT: PAMELA A. GUERRERIO, NIAID
Pamela Guerrerio has found that exposure to peanuts through the skin can prime the development of ps-Teff cells, which promote sensitization to peanuts, despite the presence of normal numbers of ps-Treg cells.
AD plays a role in the development of food allergies (FA), too. Over the past few decades, both AD and FA haved increased in the prevalence. “More evidence is suggesting that the pathogenesis may be linked because they often co-occur,” Guerrerio said. “Up to 66 percent of children with food allergies also have atopic dermatitis and up to 40 percent of children with atopic dermatitis have food allergies.” Regulatory T cells (Tregs) play a central role in the development of tolerance to foods—in mice—but it’s not clear what happens in humans. Guerrerio decided to explore how Tregs are linked to the development of peanut allergy (PA). She and colleagues analyzed blood samples from children with and without PA or peanut sensitivity and determined that exposure to peanuts through the skin can prime the development of peanut-specific (ps) effector T cells (Teff), which promotes sensitization to peanuts, despite the presence of normal numbers of ps-Treg cells.
In another study (which involved 77 participants, ages 2 to 20 years old) that investigated how moderate to severe AD and co-existing FAs affect the growth of children, Guerrerio found that children with AD and FAs (especially to milk) exhibit impaired growth, whereas those with AD alone are often overweight or obese.
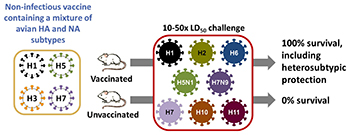
CREDIT: JEFFERY TAUBENBERGER, NIAID
NIAID scientists, led by Jeffery Taubenberger, administered a possible universal flu vaccine containing a noninfectious cocktail of four bird influenza viruses (left) to mice and ferrets. The animals received either the real vaccine or a mock vaccine (saline solution) and were later exposed (center) to eight different strains of flu via a “10-50x LD-50” challenge (50 days after vaccination or mock vaccination, the animals received 10 times the 50 percent lethal dose). All the vaccinated animals survived because the vaccine offered broad protection against many different strains of influenza including strains not represented in the vaccine (right). Next year, the scientists plan to conduct clinical trials at the NIH Clinical Center to determine whether the vaccine will offer the same kind of broad protection to people.
On the influenza front, Taubenberger noted that 2018 is the 100th anniversary of the deadly 1918 influenza pandemic that killed an estimated 50 to 100 million people in a few months. “It was probably the worst single natural disaster in all of human history,” he said. “That virus mutated and was genetically updated by reassortment to become the annual recurrent seasonal flu strains we have today.”
In the mid-1990s, Taubenberger’s laboratory at the Armed Forces Institute of Pathology (Washington, D.C.) genetically sequenced and reconstructed the virus based on preserved lung tissue from several 1918 victims (including one buried in the permafrost in northern Alaska) and studied it in high-containment labs to model its pathogenicity.
When Taubenberger came to NIAID in 2006, he set up an influenza lab group that uses both basic-research and clinical-research approaches to study not only the 1918 virus but also bird flu, swine flu, new pandemics, and seasonal influenza. His team has been using what it has learned about the pathogenicity of the 1918 flu to guide the development of universal influenza vaccines. His lab is beginning an initial clinical evaluation of these vaccine candidates in phase 1 studies at the NIH Clinical Center. Taubenberger’s investigations are shedding light on the emergence, evolution, and severity of influenza pandemics as well as of seasonal influenza.
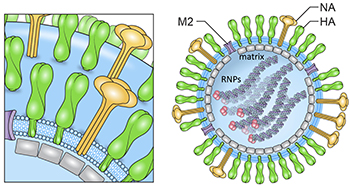
CREDIT: AUDRAY HARRIS, NIAID
Schematic of influenza virion and influenza virus organization and hemagglutinin (HA) structure. The viral glycoproteins are hemagglutinin (HA, green), neuraminidase (NA, yellow), and matrix 2 (M2) (purple). The membrane is shown in light blue. Genomic ribonucleoprotein complexes (RNP) filaments are inside with a trimeric viral polymerase complex (pink).
The structural biology of influenza and other viruses is being examined in Audray Harris’s lab. Using biochemical analyses, cryoelectron microscopy methods, and image analysis, his team determined the composition and 3-D structural organization of influenza virus–like particles of the 1918 flu. It’s important to understand flu’s molecular structure, Harris noted, because it may provide clues that will help scientists develop a universal flu vaccine. Current flu vaccines target the head region of hemagglutinin (HA), which is responsible for binding the virus to cells in the upper respiratory tract and elsewhere. But the HA subtype is changing constantly, so vaccines need to be updated annually to keep up. Even then there’s no guarantee that they will be effective against all strains. Scientists need a better strategy to protect against all versions of the virus and are beginning to target the HA-stem region, which doesn’t change as much.
To increase the immunogenicity of the HA-stem region, Harris’s group is designing nanoparticles that will have the correct structure to target the stem HAs. Structure-guided information from electron microscopy, he said, should be integrated into pipelines for the development of more efficacious seasonal and universal influenza vaccine antigens. The lessons learned from influenza vaccine electron-microscopic research could aid in the development of new vaccines for flu and other pathogens.
To watch a videocast of the “Plenary III: Food and Skin Allergies, and Virus Pathogenesis and Vaccine Development” session, which took place on Friday, September 14, 2018, go to https://videocast.nih.gov/launch.asp?26045.
This page was last updated on Wednesday, April 6, 2022
