From the Annals of NIH History: An Oral History of a Machine
If Only Our Machines Could Talk
An “Oral History” of FACS II
Gordon Margolin is a retired internist and nephrologist. Before moving to Bethesda, Maryland, in 2010, he was the director of medicine at Cincinnati Jewish Hospital and a professor of medicine, specializing (in later years) in geriatrics, at the University of Cincinnati. In 2011, he began volunteering in the Office of NIH History and Stetten Museum. He conducts oral histories with accomplished scientists. On a recent visit to the Stetten Museum storeroom, he wondered, “Wouldn’t these instruments also have great stories to tell? Let’s give it a try!” Below is his interview with the machine known as FACS II, a fluorescence-activated cell sorter.
Gorgon Margolin: Hello, Mr. FACS II. You have been part of our collections in the NIH Stetten Museum since 1992, when you were donated by Susan Sharrow, your operator in the National Cancer Institute’s (NCI’s) Experimental Immunology Branch (EIB). May I pick your memory stores for our historical collection?
FACS II: Sure! I’ve been waiting for 26 years for this opportunity, just parked here in storage and mulling over my relevance to the scientific developments of NIH and beyond. Let’s do it!
G.M.: What does FACS stand for, and what did you do?
FACS II: FACS stands for fluorescence-activated cell sorter. I was designed using laser technology, which makes the cells fluoresce brightly, and inkjet-printer technology, which provides my droplet-sorting ability. I can sort cells according to fluorescent tags attached to their surfaces. In 1969, Leonard Herzenberg at Stanford University in Stanford, California, created the first instrument that used fluorescence labeling to rapidly separate, count, and identify single live cells from a mixture of cells. (By the way, Len worked at NIH in the 1950s before he took the position at Stanford.) His machine could sort up to 1,000 cells per second. This technology, also known as flow cytometry, was an extension of single-cell analysis based on physical characteristics that had been applied in hematology and cytopathology in the 1930s. I’m sure you remember the Coulter counter (evaluated at NIH in the 1950s), which was an early instrument used to identify and count the various cells in the blood of your patients.
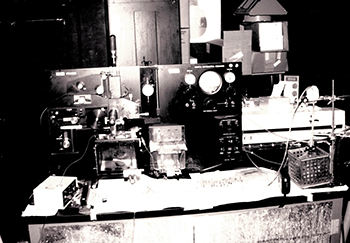
CREDIT: OFFICE OF NIH HISTORY
The FACS II machine.
G.M.: Yes, I do remember that. How did you get to NCI’s EIB?
FACS II: Early in the 1970s, some members of NCI’s intramural research program decided that my special abilities could be useful in cancer and immunological studies. Ultimately, Becton-Dickinson created me—a two-laser FACS—and delivered me to NCI in 1976 to replace a 1973 FACS I machine that was based on the Herzenberg prototype. Boy, did I ever get busy. At that time, NIH had the only immunology laboratory—outside of Stanford University—that operated a laser-based flow cytometry cell sorter. I was made available to investigators from throughout the NIH and elsewhere on the entire East Coast. Of course, using me required lots of new studies, particularly ones that sought to learn about the surface molecules of different cells. Being able to identify appropriate fluorescent tags allowed me to separate selected cells from the mixtures. The EIB group, especially Susan Sharrow, undertook these developments and guided and controlled my accomplishments.
G.M.: You went through some changes over the years. Tell me about them.
FACS II: Oh, gosh. Many of the improvements made to me were technical. I was the first two-laser FACS that Becton-Dickinson made. I could measure three to four colors of fluorescence. Later I was modified on-site to include a third laser that allowed me to measure six colors. Modern FACS machines (known as flow cytometers) now have six or more lasers and can measure at least 30 colors of fluorescence.

CREDIT: OFFICE OF NIH HISTORY
The laser-based FACS II, which arrived at NIH in 1976 and was retired to the NIH Stetten Museum in 1992, was the first flow cytometer to analyze the cell cycle in yeast, study and sort cells from a mouse brain, and analyze fluorescent liposomes and their interactions with cells. Among the other cells the FACS II sorted and analyzed were T cells, B cells, dendritic cells, stem cells, and cancer cells from bone marrow, spleen, thymus, blood, skin, brain, and other organs.
G.M.: How was your data displayed?
FACS II: When I was delivered to the NIH in 1976, my data output was displayed on an oscilloscope and captured on Polaroid instant film so it could be analyzed. NIH computer engineers later designed and built a computer interface for me so that my data could be collected and stored in a digital format. Computer programmers wrote software to analyze the data. Stanford scientists were designing interfaces around the same time, but the manufacturers didn’t provide computers with flow cytometers until the mid-1980s. Also, NCI scientists invented the Texas Red fluorescent dye to use with my second laser.
G.M.: Can you tell me in what areas you were particularly productive, and cite some examples that proved important?
FACS II: Because my technology was so new, almost every experiment I did revealed something to the scientists who used me. Our group published the first report that immune-cell subpopulations and maturity could be identified by the intensity of the fluorescent-labeled antibodies used to tag cells before FACS sorting. I was the first flow cytometer to analyze the cell cycle in yeast, study and sort cells from a mouse brain, and analyze fluorescent liposomes and their interactions with cells.
My work resulted in over 135 peer-reviewed publications. Studies included cell separation and/or analyses of mouse, rat, monkey, and human cells from individuals that were normal, had cancer, suffered from immune-related diseases, or were undergoing therapy. I sorted and/or analyzed T cells; B cells; dendritic cells; Langerhans cells; many other immune cells subsets and stem cells; and glial cells and cancer cells from bone marrow, spleen, thymus, blood, skin, brain, and other organs. I get tired thinking of all that work!
G.M.: Goodness! How much could you do in a day?
FACS II: I could sort about 2,000 cells per second, which translates to more than 500 million individual cells in eight hours. (My work day was usually more than eight hours, though.)
G.M.: How big was a sample?
FACS II: One sample could be anywhere from 10,000 cells to hundreds of millions of cells. At the time when my data output had to be captured by Polaroid instant photographs, I could analyze only about a dozen samples. Once I could send the data to a computer, however, I could analyze 100 samples in a day. Modern machines can sort 10,000 cells per second and can analyze more than a thousand samples a day.
G.M.: So why were you retired?
FACS II: Good question. I was very durable, averaging only one day down annually. But it was difficult to modify me and add new features. The EIB scientists wanted the increased speed and capabilities provided by digital technology. I guess “aging issues” and development of new technology catch up with all of us machines, but I am proud that I was able to contribute for 16 years.
G.M.: Other than your opportunity to advance scientific knowledge, what other contribution to NIH have you made?
FACS II: John Wunderlich, the NCI principal investigator who supervised the FACS laboratory in EIB, decided that this unexplored technology would best be developed by allowing all NIH investigators and outside scientists free and open access to the FACS. In 1976, this policy was quite a radical idea. The EIB FACS laboratory might have been one of the first core facilities at NIH.
Sharrow worked with interested investigators to operate me and guide my accomplishments. Some of the same investigators who used me early on eventually established new FACS laboratories in the NCI and in the National Institute of Allergy and Infectious Diseases as well as in more than 50 institutions around the world. The simple idea of open access not only promoted more rapid development of the technology, but it also allowed many scientists to work more closely together and to cross the artificial boundaries of separate labs, branches, laboratories, and institutes.
G.M.: Is there anything else you’d like to say?
FACS II: I am confined here in storage with hundreds of other devices that contributed in earlier years to major scientific advances at NIH. Maybe someday I and the others could be displayed. This would remind our scientists not to discard their retiring instruments. They should be donated to the NIH Stetten Museum so they can be kept for future use for similar “histories” or exhibits here on the campus. I feel honored that my value has been properly recognized and recorded. I thank you for this opportunity to respond to your queries and to reminisce about my contributions to NIH and to science at large.
Acknowledgements: Many thanks to Stetten Museum Curator Michele Lyons and to NCI Staff Scientist and EIB Flow Cytometry Facility Manager Susan Sharrow for their help with this article. Lyons provided provenance information regarding FACS II. Sharrow, whose continuous involvement in flow cytometry spans almost 45 years, was kind enough to confirm and expand the FACS-II story.
This page was last updated on Wednesday, April 6, 2022
