Pioneering Gene Therapy for Radiation-Induced Dry Mouth
Ongoing Clinical Trial Explores Saliva-restoring Gene Transfer
For most individuals who survive head and neck cancers, the relief of successful treatment can be tempered by a troubling side effect of some cancer therapies: chronic dry mouth. Because radiation treatment kills both tumor cells and healthy saliva-producing cells, saliva production can dwindle or even shut down completely. People with dry mouth may find it hard to speak or chew, taste, and swallow food. They may have pain from slow-to-heal ulcers and be at increased risk for tooth decay and other infections. Treatments can help, including saliva substitutes, salivary stimulants, and ice chips that moisten the mouth. But there’s no cure for radiation-induced dry mouth.
“On a clinical level, you get tired of patting somebody on the back and saying, ‘There’s nothing more I can do for you,’” said Bruce Baum, who retired from the National Institute of Dental and Craniofacial Research (NIDCR) in 2011 and remains an emeritus scientist at the institute.

PHOTO BY HWEI L. ONG, NIDCR
About three decades ago, NIDCR emeritus scientist Bruce Baum began exploring experimental treatments to repair salivary glands damaged by radiation treatment. He came up with the idea of using a modified virus to deliver a corrective gene directly into the damaged gland to restore the flow of saliva.
Frustrated by his inability to provide lasting relief for such patients, Baum began exploring experimental treatments to repair damaged salivary glands about three decades ago. By the early 1990s, the concept of gene therapy—giving patients working versions of genes to correct a disease-causing defect—was gaining traction as an experimental approach to treating diseases such as cancer and rare genetic conditions.
Inspired by a colleague’s success in transferring and expressing genes in rat lungs in 1991, Baum thought that gene transfer might also work in the salivary gland, which is biologically similar to the lungs. He came up with the idea of using a modified virus to deliver a corrective gene directly into the damaged gland to restore the flow of saliva. But the journey from concept to clinical trial would take many years and multiple studies to complete.
Baum’s work is among many parallel efforts in the field of gene therapy, where safety and logistical concerns have delayed the clinical debut of several promising approaches. Only in the past year have a handful of these therapies—two for blood cancers and one for an inherited form of blindness—received U.S. Food and Drug Administration approval. The therapy for blindness is the only one in which a corrective gene is directly administered to the patient. This so-called in vivo delivery method is the approach Baum and colleagues have been developing over 25 years to treat radiation-induced dry mouth. Their experimental therapy was the first-ever salivary-gland gene therapy tried in humans. The treatment worked better than expected, encouraging the investigators to explore additional applications for its use. Now, a second NIDCR clinical trial is underway to assess the safety and effectiveness of a similar approach using an improved gene-delivery vehicle.
From concept to clinic: A working salivary gland resembles a bunch of grapes, with globe-shaped structures made of acinar cells linked to stemlike duct cells. Water from the bloodstream flows across the acinar cells through pore-forming proteins called aquaporins located in the cell membranes. Water mixes with various proteins secreted from the cells, creating saliva that can be transported via the ducts into the mouth. Radiation damages or kills acinar cells, leaving mostly duct cells, which lack aquaporins and can’t secrete water.
Baum guessed that delivering a gene for one of the pore-forming proteins, called aquaporin 1, into damaged salivary glands might enable existing cells to transport water and restore saliva production. He chose a genetically altered adenovirus as the gene-delivery vehicle, or vector. When infused into the salivary gland, the viral vector deposits aquaporin DNA into glandular cells and instructs them to start producing the protein.
Through the 1990s and early 2000s, Baum and his colleagues worked to develop and test the concept. NIDCR virology expert John Chiorini was brought on in 1998 to optimize the viral vector. The team eventually showed that the aquaporin 1 gene therapy worked to restore saliva flow in radiation-damaged glands of rats and miniature pigs. The next step was to test it in humans.

PHOTO BY SANDRA AFIONE, NIDCR
NIDCR virology expert John Chiorini was brought on in 1998 to optimize the viral vector. The gene-transfer approach to treating radiation-induced dry mouth worked in animals and has been successful in clinical trials, too.
“No one had tried gene therapy in the human salivary gland. It was a new frontier with unknown risks,” said Chiorini, now the chief of NIDCR’s Adeno-Associated Virus Biology Section. “It wasn’t clear whether the human salivary gland would function the same way as the animal models. We intended to do a proof-of-concept trial in patients with radiation-induced dry mouth just to see if the gene transfer even worked in humans.”
Once the researchers received FDA approval for a clinical trial, the first patient was treated in 2008.
In 2012, the group published their initial results (Proc Natl Acad Sci U S A 109:19403–19407, 2012). Of the 11 participants, five had increased saliva flow and reduced sensation of dry mouth, responses that peaked in the weeks after the gene transfer. Remarkably, and to the great surprise of the investigators, the improvements in these patients persisted for several years after the one-time treatment. This outcome convinced the team that their concept could work and even bring long-lasting relief to some people.
Expanding horizons: The researchers then designed a second clinical trial using a different gene-delivery vehicle based on an adeno-associated virus. The newer viral vector was designed to transfer the same aquaporin 1 gene to the salivary glands of cancer patients with radiation-induced dry mouth.
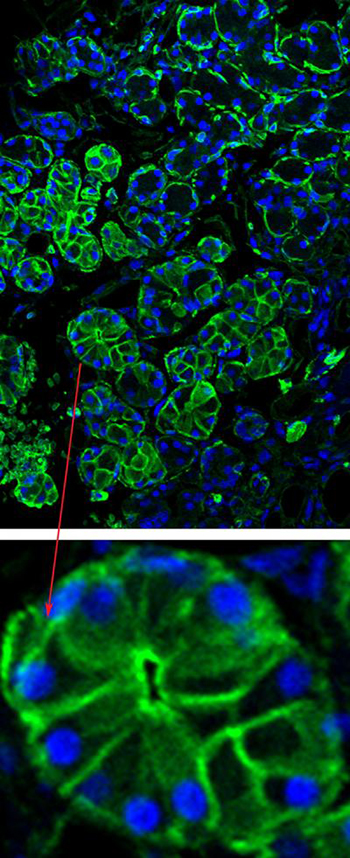
IMAGE BY CHANGYU ZHENG, NIDCR
When injected into the salivary gland, the adeno-mediated virus deposits aquaporin DNA into glandular cells and instructs them to start producing the aquaporin protein (green staining, top and bottom inset).
“We think the patients who didn’t respond in the first trial developed an immune response to the adenovirus,” said Chiorini. “Although they experienced virtually no adverse objective or subjective effects, at a microscopic level, it looks like their bodies rejected the viral vector or cells transduced by the vector.” Adeno-associated virus is thought to be less likely to elicit immune responses and to have longer-lasting effects than the previously used adenoviral delivery vehicle.
The new clinical trial began in July 2016, with four patients treated to date. So far, there’s been no sign of an immune response. Chiorini and his colleagues expect to continue enrolling participants over the next year or so, with the aim of treating up to 17 additional participants who have radiation-induced dry mouth (see “Volunteering for the Clinical Study” below).
For Baum, the first trial’s unexpected success was the realization of a dream many scientists can only hope for. “To see this go from a pie-in-the-sky idea to helping patients, I couldn’t have asked for anything more from my career,” he said. “I felt like I could retire from research because I’d achieved what I set out to do.”
There’s evidence the experimental therapy could help an even broader group of patients. Chiorini and colleagues have been studying Sjögren syndrome, an autoimmune disease that causes dry eyes and dry mouth, among other symptoms.
“Our research suggests that at least some Sjögren’s patients have a similar defect as those with radiation-damaged glands: the inability to move water,” Chiorini said. The team validated the idea in an animal model and will soon launch a clinical trial to test aquaporin 1 gene therapy in Sjögren patients. If it succeeds, the treatment could provide a path to relief for many more people suffering from dry mouth and may have even wider implications.
“Everything we’re learning from these studies can benefit the broader community of scientists who are trying to advance gene therapy as a platform for treating many kinds of genetic and acquired diseases,” Chiorini said. “As an NIH investigator, it’s a privilege to take part in high-risk, conceptual, pioneering science that might ultimately change patients’ lives.”
This article appeared in the NIDCR Science Briefs and is reprinted with permission.
Volunteering for the Clinical Study
NIDCR is seeking clinical trial volunteers, ages 18 and older, who have dry mouth after receiving radiation therapy for head and neck cancer. The goal of the study is to examine the safety and effectiveness of a gene-therapy method designed to increase saliva production. Participation requires a three-day hospital stay at the NIH Clinical Center in Bethesda, Maryland, and 10 outpatient visits over three years. Details here.
References
B.J. Baum, I. Alevizos, C. Zheng, A.P. Cotrim, S. Liu, L. McCullagh, et al., “Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction,” Proc Natl Acad Sci U S A 109:19403–19407 (2012); DOI:10.1073/pnas.1210662109.
I. Alevizos, C. Zheng, A.P. Cotrim, S. Liu, L. McCullagh, B.J. Baum, et al., “Late responses to adenoviral-mediated transfer of the aquaporin-1 gene for radiation-induced salivary hypofunction,” Gene Ther 24:176–186 (2017); DOI:10.1038/gt.2016.87.
Z. Lai, H. Yin, J. Cabrera-Pérez, M.C. Guimaro, S. Afione, D.G. Michael, J.A. Chiorini, et al. “Aquaporin gene therapy corrects Sjögren's syndrome phenotype in mice,” Proc Natl Acad Sci U S A 113:5694–6599 (2016); DOI: 10.1073/pnas.1601992113.
A. Corden, B. Handelman, H. Yin, A. Cotrim, I. Alevizos, and J.A. Chiorini, “Neutralizing antibodies against adeno-associated viruses in Sjögren's patients: Implications for gene therapy,” Gene Ther 24:241–244 (2017); DOI:10.1038/gt.2017.1.
This page was last updated on Friday, April 8, 2022
