WHIM Syndrome
Whimsically Cured by Chromosome Shattering
It was a medical mystery that only the NIH could solve: How a woman was spontaneously cured of a rare disease more than 20 years ago after first being diagnosed. Researchers at the NIH recently unraveled this mystery cure in the journal Cell.
In 1964, a nine-year-old girl was featured in two case studies in the New England Journal of Medicine as the first person ever diagnosed with myelokathexis, the inability of certain white blood cells to leave the bone marrow, enter the bloodstream, and fight infection (N Engl J Med 270:699–704, 973–979, 1964). This extremely rare inherited immunodeficiency disease later came to be known as WHIM syndrome [warts, hypogammaglobulinemia (low concentrations of immunoglobulins), infections, and myelokathexis (white blood cells trapped in the bone marrow)]. In 2003, researchers found that the syndrome is caused by mutations in the chemokine receptor gene CXCR4, which is expressed on most white blood cells (Nat Genet 34:70–74, 2003).
Two years ago, a 58-year-old woman contacted the National Institute of Allergy and Infectious Diseases (NIAID), where a team of scientists was studying WHIM syndrome and CXCR4. She said that she and two of her three daughters, ages 21 and 23, wanted to be evaluated for WHIM syndrome. It turned out that the woman had been that nine-year-old girl and that the disease had disappeared when she was in her 30s.
Of the 60 known individuals with WHIM syndrome, 29 of whom are patients at the NIH, there had been no record of a patient recovering. The researchers, led by Philip M. Murphy, chief of NIAID’s Laboratory of Molecular Immunology, were intrigued and began a quest to unravel this medical mystery.
NIAID
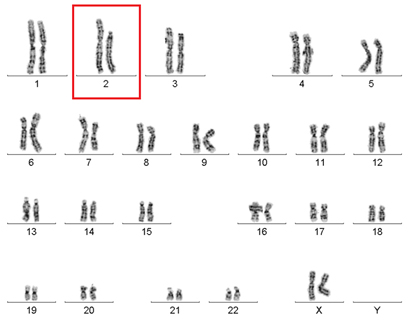
NIAID researchers discovered why a woman with WHIM syndrome was spontaneously cured of the disease. Her white blood cells were missing a large portion of chromosome 2, where the mutated WHIM allele resides. In essence, the patient had deleted the bad copy of the gene and was now cured. Shown: The above chromosome pairings, which are from her white blood cells, show that one copy of chromosome 2 (in box) is significantly shorter than the other.
First, the team took blood samples to test for the genetic mutation in CXCR4. The daughters’ blood tested positive for the mutation, but their mother tested negative. The researchers hypothesized that the mother was a genetic mosaic—a condition in which cells within the same person have a different genetic makeup—and only some of her cells carried the mutated gene. To validate this hypothesis, the scientists repeated the genetic screen for CXCR4 in epithelial cells and fibroblasts, which tested positive for the mutation.
The team followed the trail to the source of blood cells—the bone marrow. They sent a bone-marrow sample for cytogenetic testing to examine the number and structure of her chromosomes. They discovered that the woman’s white blood cells were missing a large portion of chromosome 2, where the mutated WHIM allele resides. In essence, the patient had deleted the bad copy of the gene and was now cured.
“At this point we were dealing with some kind of wild genetic instability we had never seen before,” said Murphy.
Murphy’s team then attended a Wednesday Afternoon Lecture Series presentation in 2013 by Frederick Alt (Harvard) on genetic instability in the immune system where they learned the name for what they were observing (http://videocast.nih.gov/launch.asp?17874). Alt mentioned that the phenomenon of chromothripsis, the shattering and rearrangement of a chromosome, is usually associated with cancer. This novel mechanism for genetic shuffling had only recently been described by other researchers (Cell 1441:27–40, 2011).
Chromothripsis might be “something that happens all the time in everyone, maybe every day in some rare cells, but it is probably so catastrophic that the cell dies,” Murphy speculated. “It’s an invisible event; you never see it [and] you can only look for it when you have a bad cancer or a good cure.”
In this case, chromothripsis resulted in a good cure. “Instead of causing cancer, it has [permitted a single] hematopoietic stem cell to take over the marrow,” explained David H. McDermott, a staff clinician in Murphy’s lab and first author on the 2015 Cell paper (Cell 160:686–699, 2015). As the new cells with the deleted gene repopulated the patient’s marrow, her symptoms vanished.
The team used whole-genome sequencing to try to understand how this one cell took over and cured the patient. They found that 17 large areas of chromosome 2 were missing and 164 genes had been deleted.
In order to focus on the deletion of the disease gene, staff scientist and co-first author Ji-Liang Gao used a mouse model in which only one copy of the Cxcr4 gene was deleted. This single genetic mutation gave hematopoietic stem cells a strong growth advantage. When transplanted into another mouse with either wild-type or WHIM model mouse bone-marrow cells, the altered cells quickly took over as they had in the cured patient.
The lab plans to try to recreate this phenomenon in patients with WHIM syndrome by using genome editing to wipe out or correct the mutant allele in affected cells and then returning them to the patient. This technique was proposed in their recent U.S. patent application (http://1.usa.gov/1DUFuo9) and ultimately could be applied to treat countless other blood diseases as an adjunct to specific gene therapy.
The miraculously cured WHIM patient, now 60, is still doing well.
“She’s got two New England Journal of Medicine papers and one Cell paper all about her,” said Murphy. “I told her that if she was an academic that she could get tenure on that alone.”
To read NIH Director Francis Collins’s blog post on this mystery, go to http://directorsblog.nih.gov/2015/03/05/shattering-news-how-chromothripsis-cured-a-rare-disease.
This page was last updated on Monday, April 25, 2022
