Research Festival: Selected Symposia 2014
Viewing the Unviewable: Microscopy Beyond the Limits of Light
Wouldn’t it be great to have X-ray specs (or some other gadget) that would let you look deep into a cell, so you could see each molecule dancing its way through the complexity of the cell membrane or cytoplasm?
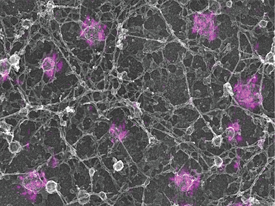
KEM SOCHACKI (NHLBI) AND GLEB SHTENGEL (HHMI); COURTESY OF JUSTIN TARASKA (NHLBI)
Justin Taraska combined super-resolution light microscopy with electron microscopy to reveal individual molecules of clathrin that are decorating endocytic pits as they form on the inside of a cell membrane.
While super X-ray specs aren’t yet a reality, researchers have been working steadily toward new and better ways to view molecules at high resolution, whether purified or in the context of the cell. A Research Festival symposium featured presentations on super-resolution light-microscopy and electron-microscopy techniques that allow the direct visualization of proteins to the nanoscale level.
To be able to see individual proteins on clathrin-coated pits on a cell membrane, Justin Taraska (NHLBI) combined the single-molecule fluorescence interference microscopy super-resolution light-microscopy technique with transmission-electron microscopy (TEM).
Richard Leapman (NIBIB) presented new electron-microscopy techniques for imaging cells and tissues in three dimensions. One of these techniques was scanning TEM (STEM), which allows for imaging of much thicker sections; he used this technique to directly visualize whole-ribbon synapses in theetina and postsynaptic densities in hippocampal neurons.
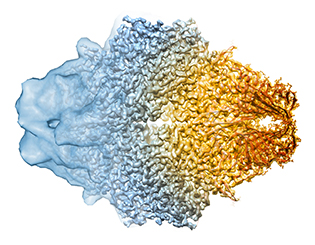
CREDIT: DOREEN MATTHIES, VERONICA FALCONIERI, SRIRAM SUBRAMANIAM, NCI
Advances in cryo-electron microscopy are letting scientists get closer to seeing the full atomic structure of protein complexes. Where once it could only show the general shape of a protein (left), now cryo-electron microscopy can reveal the fine details (center), letting scientists tease out models for the protein’s structure (right). The protein shown here is the bacterial enzyme beta-galactosidase.
Jordan Beach (NHLBI), a FARE Award winner, used a combination of total internal-reflection fluorescence and structured-illumination microscopy to view the movement of different myosin isoforms and determine how these isoforms interact.
Greg Alushin (NHLBI) used cryo-TEM and helical reconstruction to visualize vinculin, a protein involved in focal adhesions, when bound to actin fibers. He found that a small portion of the vinculin molecule moves, potentially revealing how vinculin interacts with the cytoskeleton.
Alasdair Steven (NIAMS) used TEM in conjunction with STEM to study encapsulin, a newly discovered bacterial complex that resembles a viral capsid. He called this complex “superferritin” for its capacity to store much more iron than traditional ferritins.
And finally, Sriram Subramaniam (NCI) closed the session highlighting recent advances that he and his colleagues have made in cryo-electron microscopic studies of purified protein assemblies. He demonstrated how they are now able to visualize protein structures at near-atomic resolution by taking advantage of new technologies for direct electron detection.
From the 2014 Research Festival session “Advances in Molecular Microscopy,” chaired by Sriram Subramaniam (NCI) and held on Tuesday, September 23, 2014.
Taking the Leap…Into Biotech Firms
Several intramural investigators who left NIH to work in the biotechnology industry, or start their own companies, shared their stories.
Bruce Weintraub spent 27 years at the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) before leaving to co-found Trophogen, Inc. (Rockville, Maryland) in 2001. While at NIDDK he cloned the human thyroid-stimulating hormone gene and recognized the importance of glycosylation (attaching carbohydrates to proteins) in hormone function. The discoveries and ideas he had at NIDDK formed the basis of Trophogen, which is developing glycoprotein hormone superagonists for human infertility and hormone-driven cancer treatments.
John Magnani co-founded GlycoMimetics, Inc. (Gaithersburg, Maryland) in 2013, after having spent 10 years at NIDDK. Like Weintraub, Magnani is interested in the role of carbohydrates in disease: GlycoMimetics is designing therapeutic compounds that mimic the structure and activity of naturally occurring carbohydrates.
Veronica Hall, who was a postdoctoral fellow at the National Cancer Institute for three years, credits NIH with inspiring and preparing her to succeed in the biotechnology industry. She joined Emergent BioSolutions (Rockville, Maryland) as a non-bench scientist specialist in 2008 and quickly rose to director of strategic development. She looks for innovative ideas, evaluates new technologies, and finds funding sources.
For six years, Scott Koenig worked on HIV and human T-cell lymphotropic virus type 1 while he was at the National Institute of Allergy and Infectious Diseases. In 1990 he joined Medimmune (Gaithersburg, Maryland) when the company was only two years old and had fewer than 40 employees. He spent 11 years with Medimmune, helping it grow as a publicly traded company, which subsequently became a subsidiary of AstraZeneca with more than 1,000 employees. The experiences Koenig gained and contacts he met while at Medimmune paved the road for him to co-found his own biotechnology company, MacroGenics, Inc. (Rockville, Maryland) in 2000. MacroGenics engineers antibodies to create therapeutic products including those with dual specificities that target multiple molecules.
These are only a sampling of NIH investigators who took the leap into entrepreneurship. If you are thinking about starting or joining a biotechnology company, heed Veronica Hall’s advice: “If you do not think you are qualified, remember that a Ph.D. is the only degree without a textbook telling you what to do and how to do it.”
From the 2014 Research Festival session “Commercial Development of My Research Discoveries: Still More Personal Stories from Former NIH Intramural Scientists,” chaired by Steven Ferguson (OD) and Todd Chappell (OD) and held on Monday, September 22, 2014.
A (Brain) Image Is Worth a Thousand Words
Imaging biomarkers that characterize neurological and psychiatric disorders are crucial for the accurate diagnosis and prognosis of brain disorders. Recent advances in neuroimaging have shown promise in identifying imaging-based biomarkers that are useful for predicting therapeutic outcomes. Several NIH researchers from different institutes presented their work at the Research Festival reflecting the latest developments in searching for imaging biomarkers of brain disorders.
Christopher Harris (a FARE winner in the National Institute of Neurological Disorders and Stroke) discussed his recent findings on the neural basis of goal-directed behavior in zebrafish. By using a genetically encoded calcium indicator called GCaMP6, he located specific neurons whose activity correlated with different types of movement including prey location and decision-making.
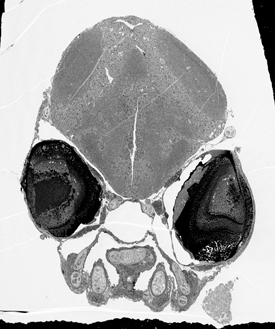
CHRISTOPHER HARRIS, NINDS
Christopher Harris (NINDS) discussed his recent findings on the neural basis of goal-directed behavior in zebrafish. Shown: cross-section through the head of a seven-day-old zebrafish. The fish was imaged in an electron microscope at 300X magnification. The two dark spheres are eyes. Above and between the eyes is the developing brain.
A different behavioral model was examined by Hanbing Lu (National Institute on Drug Abuse, NIDA) using functional magnetic resonance imaging (fMRI) in a rodent model of addiction. This study showed that after cocaine self-administration by a rodent, its brain undergoes an increase in plasticity specifically in the prefrontal cortex, anterior insular cortex, and dorsal striatum, which can serve as potential biomarkers at the circuit level.
Several other investigators also use fMRI to measure brain activity. Yihong Yang’s (NIDA) group did a study that emphasized the importance of brain network coupling (interactions between large-scale brain networks) in tobacco dependence: They found that brain-network coupling predicts acute nicotine abstinence-induced craving and cognitive deficits in smokers. Allison Nugent (National Institute of Mental Health, NIMH) described how fMRI scans revealed neural correlates underlying ketamine’s ability to provide faster relief than typical antidepressants in patients with severe depression.
James Blair (NIMH) uses fMRI to understand which brain regions are activated in different types of conduct disorder, a childhood behavior disorder characterized by aggressive and destructive activities such as defiant or impulsive behavior, drug use, and criminal activity. He hopes his findings will enhance diagnosis and help with treatments.
Karen Berman (NIMH) also uses fMRI to decipher neural abnormalities in Williams syndrome, a rare neurodevelopmental disorder characterized by cardiovascular disease, developmental delays, deficits in visuo-spatial processing, and a striking overly friendly personality. She found that people with Williams syndrome had decreased gray matter in the part of the brain linked to visuo-spatial processing, and they had abnormal structure and function in parts of the brain linked to social and emotional processes.
From the 2014 Research Festival’s session “Searching for Imaging Biomarkers of Brain Disorders: From Preclinical Models to Clinical Treatments,” chaired by Yihong Yang (NIDA) and held on Wednesday, September 24, 2014.
DeMystifying Medicine: Connecting the Dots
Win Arias (Clinical Center) has a knack for connecting the dots between advances in biology and their ultimate applications in the clinic. In 2001, he launched a program that does just that: the Demystifying Medicine seminar series, a course that features presentations by basic scientists, clinicians, and patients on major diseases and health conditions. This year for the first time, Arias offered Research Festival goers a sampling of his ever-popular program in hopes of enticing people to register for the 2015 session, which begins in January (http://demystifyingmedicine.od.nih.gov).

Win Arias likes to use this image of the Brooklyn Bridge to represent his DeMystifying Medicine program, which is designed to help bridge the gap between advances in biology and their application to major human diseases. Each session includes clinical and basic-science components presented by NIH staff and invitees. For more information, go to http://demystifyingmedicine.od.nih.gov.
Craig Blackstone (National Institute of Neurological Diseases and Stroke) and William Gahl (National Human Genome Research Institute) are searching for answers to diseases of unknown etiology. Blackstone presented research on people with hereditary spastic paraplegias, a group of inherited disorders that are characterized by progressive weakness and spasticity (stiffness) of the legs. Using genetic sequencing, he was able to find mutations in a particular protein complex and subsequently uncovered how these proteins function in cells.
Gahl, who’s the director of the NIH Undiagnosed Diseases Program (UDP), also used genetic sequencing to discover the causes of some rare diseases. The UDP has been so successful that the NIH Common Fund started an Undiagnosed Diseases Network to encourage and train clinicians across the country to use genomic data to diagnose diseases.
Then National Cancer Institute’s Frank Maldarelli and John Coffin talked about their research on the human immunodeficiency virus (HIV) and antiretroviral therapy (ART) and how to respond to changes in the virus and the host.
Maldarelli presented an overview of HIV and its phylogeny, including the finding that HIV-1 is related to the simian immunodeficiency virus found in chimpanzees and that most likely humans first became infected by coming into contact with infected chimpanzee meat. Although 26 antiretroviral drugs can prolong life and prevent mother-to-child transmission of HIV, new cases of HIV infection still occur. Research on HIV is not nearly finished, he said.
Coffin explained why ART can only hold HIV infections at bay, but not cure them. ART blocks the formation of provirus (the virus genome that is integrated into the DNA of a host cell), but it has no effect on already-infected cells. In fact, should ART therapy be interrupted for as little as a week (even after years of therapy), those infected cells can cause the concentrations of HIV to return to pretherapy levels.
From the 2014 Research Festival session “Demystifying Medicine: A Sampler,” chaired By Win Arias (CC) and held on Tuesday, September 23, 2014.
The Challenges of Aging
Life expectancy is on the rise, but increased life span brings with it an increased burden of chronic disease and disability. Luckily, geriatric epidemiologists have made invaluable contributions to our understanding of the health status and functional trajectory of older individuals and have helped generate interventions to improve their lives. Several NIH researchers highlighted their aging-related research.
National Institute on Aging (NIA) Scientific Director Luigi Ferrucci argued for the need to “characterize and measure aging parameters and establish a list of candidate measures” in order to better understand disease and disability in the elderly and why frailer individuals are at a higher risk of developing comorbidity and disability.
“Age is the strongest predictor for most chronic diseases,” said Andrew Johnson (National Heart, Lung, and Blood Institute), who focuses on how the genetic and genomic underpinnings of individuals with cardiovascular disease affect their response to therapy. Using gene-expression studies, his team has predicted the “top 50 to 100 genes” identified with aging.
Michelle Shardell (NIA) described a new method to handle selection bias and incorrect conclusions in epidemiologic studies giving as an example a case study of vitamin D and physical performance.
Charles Matthews (National Cancer Institute, NCI), who studies the relationship between physical-activity behaviors and cancer risk, is developing measurements that will better define the type and amount of physical activity needed to reduce the risk of developing cancer. This knowledge is critical for evidence-based public-health guidelines linking physical activity to cancer prevention and control.
FARE Award winner Elizabeth Yanik (NCI) presented research, based on an analysis of 2002–2009 Medicare claims and other data, that suggested that oral leukoplakia (white or gray plaques inside the mouth) is an early indicator for the risk of oral-cavity cancer in elderly adults. The earlier the diagnosis, she reported, the better a patient’s chance of survival.
From the 2014 Research Festival session “Epidemiology of Aging,” co-chaired by Francesca Macchiarini (NIAID) and Evan Hadley (NIA) and held on September 22, 2014.
Oh, the Pain of It All
Research Festival attendees heard from several NIH researchers who are conducting pioneering pain research.
“Pain and depression frequently co-exist,” explained Ted Usdin (National Institute of Mental Health), who found that, in mice with a temporary sciatic-nerve injury, depression and anxiety—as well as cellular changes in the hippocampus—persist for at least 30 days after the pain is gone. There seems to be signaling coming from somewhere other than the original pain source, said Usdin. “We are trying to figure out where.”
To figure out the neurobiology of pain, Kathryn Tabor (FARE Award winner in the National Institute of Child Health and Human Development) is using electric pulses to activate certain neurons that drive the rapid-escape response in zebrafish. The rapid-escape response is similar to the withdrawal reflex exhibited by higher animals in response to a pain stimulus.
Other researchers, such as Michael Iadarola (Clinical Center), are trying to figure out how to reduce pain. Iadarola described a clinical trial that is testing whether resiniferatoxin (RTX), a naturally occurring chemical from a cactus-like plant, can reduce the need for the opioids that are typically used to control certain types of pain. RTX works by destroying nerves that transmit pain information. “It’s too early to draw conclusions,” about the drug’s effectiveness, he said.

Bob Stockfield; Courtesy: NCCAM
Pain researchers in the National Center for Complementary and Alternative Medicine have found that not only do yoga practitioners tolerate pain more than twice as long as individually matched control subjects, but they also have more gray matter in multiple brain regions.
Scientific Director Catherine Bushnell (National Center for Complementary and Alternative Medicine) wrapped up the session by describing her institute’s focus on understanding the brain’s role in perceiving, modifying, and managing pain, with a special emphasis on nonpharmacological modulation of pain. She described one study that explored the mechanisms underlying reduced pain perception in yoga practitioners. Not only do yogis tolerate pain more than twice as long as individually matched control subjects, but they also have more gray matter in multiple brain regions. Bushnell also mentioned that other NCCAM researchers are exploring how, in mice, exercise and environmental enrichment may play roles in reducing the perception of pain.
From the 2014 Research Festival session “Moving Molecules from Model Systems to Medicine in Pain Research,” co-chaired by Alexander Chesler (NCCAM) and Yarimar Carrasquillo (NCCAM) and held on Tuesday, TueSeptember 23, 2014.
Nature’s Drugs
We may never be able to curb Nature’s destructive energy that produces terrible storms, earthquakes, and typhoons, but scientists may have found a way to harness her ability to produce compounds that can fight infection, alleviate pain, and even battle cancer.
“By collecting rare organisms, we can find new chemical scaffolds with promising antibacterial activity,” said NIDDK senior investigator Carole Bewley, who extracts antibiotic-like chemicals from rare marine organisms. “In fossil sponges we found a derivative that kills gram-negative bacteria,” many of which are resistant to several classes of antibiotics. Her lab recently discovered a rare species of algae that produces a substance that inhibits dangerous infections such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus faecium.
FARE winner Sabrina Lusvarghi, a postdoc in Bewley’s lab, talked about her work with the potent human immunodeficiency virus inhibitor griffithsin, a protein isolated from red algae. She is helping to design glycopeptides (a type of antibiotic drug) that mimic such natural products.
Jay Schneekloth (NCI-CCR) is identifying natural products that can inhibit protein SUMOylation (a post-translational modification that regulates transcription-factor activity). The aberrant regulation of SUMOylation is linked to several cancers. He has used a high-throughput screen of natural-product extracts to identify 10 naturally occurring small molecules that can inhibit SUMOylation. But, he cautioned, “With natural products…you have to be careful what you’re working with.”
NCCAM investigator Alexander Chesler is certainly careful with what he’s working with—poisonous snake venoms. He is sure they hold a key to understanding, and possibly alleviating, pain. Depending on the snake, the venoms can either activate or block ion channels (tiny pores that allow the flow of ions across cell membranes) in humans and other animals. Texas coral snake venom, for example, activates a particular ion channel and causes pain, while venom from the black mamba snake blocks the same ion channel and acts as an analgesic.
Christina Annunziata (NCI-CCR) is studying the role of nuclear factor kappa–b (NF-κB, a naturally occurring transcription factor) signaling in ovarian cancer. Her lab defined a gene-expression signature that reflected increased NF-κB signaling in ovarian cancer cell lines and increased the cancer’s aggressiveness. She hypothesizes that appropriate molecular testing will identify patients with the highest NF-κB activity and that they would be most responsive to an investigational drug she’s working on and looks forward to collaborating on a screen of the NIH natural product library to enhance efficacy of NF-kB blockade.
From the 2014 Research Festival session “New Biological Targets for Drug Discovery/Development,” chaired by John Williamson (NCCAM) and held on Monday, September 22, 2014.
Let’s Light Up the Brain
Optogenetics, a revolutionary tool that integrates optics and genetics, uses pulses of light to drive or inhibit the firing of neurons. One Research Festival symposium featured presentations by several scientists who are using the technology to illuminate the workings of the brain.
FARE winner Kimberly LeBlanc (National Institute of Diabetes and Digestive and Kidney Diseases, NIDDK) demonstrated the role of different neuron subtypes in the striatum, the part of the brain that coordinates motivation with body movement in anxiety. By optogenetically activating the striatum’s direct and indirect pathways in mice, she demonstrated that the indirect pathway is more involved in promoting anxiety.
Hadley Bergstrom (a FARE winner in the National Institute of Alcohol Abuse and Alcoholism) is also studying the striatum. In particular, he is using optogenetics in mice to determine how two sections of the dorsal striatum mediate reward-based learning. By experimentally silencing neurons in the two sections, he found that learning is faster if the dorsolateral section is silenced.
Central pattern generators (CPGs) produce rhythmic oscillations in the spinal cord, and these oscillations coordinate movement. Melanie Falgairolle (National Institute of Neurological Disorders and Stroke) uses an ex vivo optogenetic technique to inactivate neurons and demonstrated that motor neurons are involved in feedback to the CPGs.
Ross McDevitt (National Institute on Drug Abuse), who uses optogenetics to target the activation of certain neurons within the dorsal raphe nucleus (located on the brainstem), may have discovered a reward pathway: He showed that a small subset of these cells project to the ventral tegmental area (located in the midbrain and involved in the reward circuitry of the brain).
Another tool for noninvasively controlling neurons is chemogenetics, commonly referred to as designer receptors exclusively activated by designer drugs (DREADD). Zhenzhong Cui (NIDDK) used DREADDs to reveal an important signaling cascade that stimulates chronic overeating and weight gain in mice.
From the 2014 Research Festival’s session “Optogenetic Approaches to Investigating the Nervous System,” chaired by Lex Kravitz (NIDDK) and held on Tuesday, September 23, 2014.
Women’s Health: Bridging the Gap between Sex and Gender and between Race and Ethnicity
People of color are predicted to make up the majority of the population in the United States by 2043, Janine Austin Clayton, director of the NIH Office of Research on Women’s Health, told the audience that had gathered to learn about health issues concerning women of color. “By 2050, 53 percent of the total U.S. female population will be women of color.”
Still, there are alarming health disparities among women of different races and ethnicities for many diseases such as obesity, cardiovascular diseases (CVD), stroke, and autoimmune diseases such as systemic lupus erythematosus. Clayton announced that NIH will soon release the fourth edition of the Women of Color Health Data Book, which summarizes key health indicators and potential historical, cultural, and socio-geo-demographic contributors. Several NIH intramural scientists presented their own related work at this symposium.

KJERSTEN BUNKER WHITTINGTON, OD
The speakers and organizers of the symposium "The Health of Women of Color: A Critical Intersection at the Corner of Sex/Gender and Race/Ethnicity." From left to right: Debbie Cohen; Marie Bernard (NIA); Cerise Elliott (NIA); Tiffany Powell-Wiley (NHLBI); Lauren Wood (NCI); Jennifer Plank (NIDDK); Salman Tajuddin (NIA); Nakela Cook (NHLBI); Gina Brown (OD); and Janine Clayton (OD). Missing: Tamara Harris (NIA)
Lauren Wood (NCI), who described her research on the treatment of cancer, believes that novel therapeutic vaccines and immune-based therapies would, one day, help overcome the challenges of cancer, HIV infection, and potentially even lupus. Gina Brown (OD), in the Office of AIDS Research (OAR), explained how a trans-NIH plan for HIV-related clinical research would contribute to an understanding of the relationship between sexual violence and HIV infections among women. Tamara Harris (NIA) presented her research on analyzing body-composition differences, sex, race, socioeconomic, and demographic data among the elderly.
Tiffany Powell-Wiley (NHLBI) pointed out how obesity and environmental factors could contribute to CVD, particularly in African-American women. But Nakela Cook (NHLBI) suggested that the monitoring of health-care data might lead to tailored interventions. Genome-wide association study analysis performed by FARE Award winner Salman Tajuddin (NIA) aims to identify a susceptibility marker for subclinical atherosclerosis among African-American women and men.
Want to know more and be involved? Marie Bernard (NIA) invites you to join NIH’s Women of Color Research Network (WoCRn) for networking, mentoring, recruitment, and career development (http://www.wocrn.nih.gov/).
From the 2014 Research Festival session “The Health of Women of Color: A Critical Intersection at the Corner of Sex/Gender and Race/Ethnicity,” co-chaired by Janine Clayton (OD) and Marie Bernard (NIA) and held on September 23, 2014.
Opening the Clinical Center’s Doors to Extramural Researchers
The NIH Clinical Center (CC) has worked with extramural investigators for years, but an expanded opportunity for extramural investigators started two years ago, and several grant-supported collaborative projects are underway. External investigators who are partnering with NIH intramural scientists—through the “Opportunities for Collaborative Research at the NIH Clinical Center (U01)” funding program—have access to the CC’s broad resources including advanced equipment, technology, and tools; biomedical specimens; rare diseases; and protocol support and unique data sets. This symposium highlighted a few of the program’s first 10 projects, which are three-year renewable awards of up to $500,000 per year (http://www.nih.gov/news/health/mar2014/nichd-13.htm).

Researchers from the National Cancer Institute (NCI) and the Children’s National Medical Center (Washington, D.C.) are collaborating on a clinical trisl for a new drug treatment to prevent relapse in a childhood leukemia called acute myeloblastic leukemia (AML). Although current therapy results in complete remission in nearly 90 percent of pediatric AML patients, nearly 50 percent of them relapse within two years. Leukemia stem cells are highly resistant to conventional chemotherapy and are likely responsible for the relapse.
Brigitte Widemann (NCI) and Reuven Schore (Children’s) talked about the preclinical and clinical development of the antibiotic echinomycin, which has been used to treat solid tumors in humans and has been found to be effective in eliminating AML stem cells in mice. The CC pharmacy has used its expertise to reformulate echinomycin so it can be fine-tuned and tested safely in clinical trials.
The National Institute of Allergy and Infectious Diseases (NIAID) and Oregon State University (Corvallis, Oregon) have teamed up to analyze intestinal microbiota after ustekinumab (an anti-interleukin-12-p40-subunit and anti-interleukin-23-p40-subunit agent) is used to treat common variable immunodeficiency (CVID) enteropathy. CVID impairs the immune system and is characterized by decreased concentrations of serum immunoglobulins, recurrent sinopulmonary infections, and, in 20 percent of patients, a noninfectious, hard-to-treat malabsorbtion syndrome known as CVID enteropathy.
NIAID’s Warren Strober and Ivan Fuss joined with NIAID postdoc alumnus Andriy Morgun (now at Oregon State) to describe their work. Morgan explained that gut microbiota play a critical role in CVID enteropathy in mice and are likely to play an important role in humans, too. Recent studies have suggested that CVID enteropathy results from a complex interaction between the gut microbiota and the intestinal epithelium, leading to a hyperactive mucosal immune response. The researchers are conducting a clinical trial to determine whether the problem could be corrected by selectively inhibiting the immune response with ustekinumab, which is already approved for the treatment of psoriasis in phase 3 trials for refractory Crohn’s disease patients.
Peter Pinto (NCI), along with other NCI and Clinical Center researchers, is collaborating with investigators at the University of Michigan Medical School (Ann Arbor, Michigan) to study the molecular and metabolic basis of prostate cancer. They are combining the advanced magnetic resonance imaging (MRI) and metabolic imaging techniques developed at NIH for prostate cancer with the integrative clinical sequencing approaches used by the Michigan Center for Translational Pathology. Patients will be enrolled in a clinical trial at NIH and their tissue samples sent to Arul Chinnaiyan’s lab in Michigan to be analyzed.
FARE winner Arvin George (NCI), who works in Pinto’s lab, described how a multiparametric MRI method may be better than traditional transrectal ultrasound-guided biopsy in identifying specific areas in the prostate that are suspicious for cancer. The researchers hope their work will lead to more effective therapies for prostate cancer, which is the second most common cause of cancer in men (after skin cancer).
In a grant funded by the National Institute for Child Health and Human Development (NICHD), Irini Manoli at the National Human Genome Institute (NHGRI)—and other investigators from NICHD, NHGRI, and the National Eye Institute—partnered with researchers at Boston Children’s Hospital (Boston) and Mount Sinai Medical Center (New York) to study the birth defect Moebius syndrome and related congenital facial-weakness disorders. Moebius syndrome is a neurodevelopmental disorder that causes facial weakness and limited eye movements. It is associated with intellectual disabilities; autism; hearing loss; difficulty swallowing and breathing; and craniofacial, limb, heart, and other abnormalities.
Manoli and Ethylin Wang Jabs (Mount Sinai) described how, with the help of the NIH Clinical Center, the team will be conducting extensive phenotype analysis on approximately 75 families with Moebius and other congenital facial-weakness syndromes. Their analysis will be followed by state-of-the-art genetic studies aimed to identify possible genetic causes underlying these syndromes. Studies to be conducted at the Clinical Center include genetic, ophthalmologic, audiological, dental/craniofacial, neurological, neurocognitive, and psychiatric evaluations; and brain-imaging studies. The researchers hope that a more comprehensive definition of the phenotypic and genotypic spectrum of these birth defects will have a significant impact on the understanding of molecular pathways underlying cranial nerve and brain-stem development, and potentially more common childhood disorders such as autism.
The next phase of grant submission—for the “Opportunities for Collaborative Research at the NIH Clinical Center (U01)”—resumes in early December with a newly established presubmission review process aiming to both expedite and broaden potential research collaborations. For more information, visit http://clinicalcenter.nih.gov/translational-research-resources/index.html.
From the 2014 Research Festival session “Opening the CC doors: Opportunities for Collaborative Research at the NIH Clinical Center (U01),” chaired by John Gallin (CC) and Constantine Stratakis (NICHD) and held on Tuesday, September 23, 2014.
This page was last updated on Tuesday, April 26, 2022
