The Epidemic That Just Won’t Quit
NIH Obesity Researchers to the Rescue
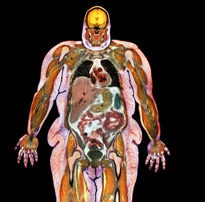
GUSTOIMAGES/Photo Researchers, Inc.
Obese woman, colored magnetic resonance imaging (MRI) scan. This coronal scan, vertically through the body, has revealed the internal organs and tissues, including the bones, muscles, and layers of fat. The brain is seen in the head, and in the torso the intestines, liver, heart and lungs are seen. Here, the liver (triangular, upper left) is enlarged and the lungs are compressed in comparison to the organs of a woman who is not overweight (not shown). The extra body weight present in obese patients puts a strain on the heart and lungs.
Obesity has become an epidemic in the United States that affects approximately one-third of adults and nearly 17 percent of children and teens. People who are obese are at increased risk for developing many serious health problems including diabetes, fatty liver disease, cardiovascular disease, and a reduced life expectancy. And obesity disproportionately affects racial- and ethnic-minority populations as well as those who are socioeconomically disadvantaged. The high prevalence of obesity is thought to result from the interaction of genetic susceptibility with behaviors and environmental factors that promote increased calorie intake and sedentary lifestyles. NIH funds and does research on all aspects of obesity. Here, we share highlights of a few of the NIH intramural researchers who are tackling the epidemic.
NIDDK Experts Tackle Obesity
The National Institute of Diabetes and Digestive and Kidney Diseases, as its name implies, is front and center in the battle against obesity, given it is a major cause of diabetes, digestive and kidney diseases. NIDDK’s obesity researchers include Kevin Hall, Monica Skarulis, Kong Chen, William Knowler, Leslie Baier, and many others.
Kevin Hall, for one, never expected his Ph.D. in physics and his expertise in mathematics modeling to lead to a career as an obesity researcher. He went from studying nonlinear dynamics in graduate school to building computer models of type 2 diabetes at a biotech company to constructing mathematical models of metabolism at NIDDK. Today, Hall is seeking to understand how changes in diet and exercise change the way a person metabolizes food. His findings may contribute to new approaches for tackling obesity.

Global Health TV/Elsevier
Kevin Hall participated in a press conference in conjunction with the Lancet series on obesity last summer.
Hall’s group of computer-modeling experts works with clinical investigators to study one of the enduring questions in obesity research: Are all calories equal whether they come from carbohydrate, fat, or protein? Their study systematically addresses whether altering overall calorie intake, and the proportions of carbohydrates and fats, can change the way a body processes food. The researchers build complex models, based on previous research, to predict the outcome of dietary modifications. Then they collaborate with clinicians in NIDDK’s Metabolic Clinical Research Unit (MCRU) in the NIH Clinical Center to test their predictions on normal weight and obese volunteers.
Results thus far have shown that reducing the proportion of fat in the diet has little effect on the rate of fat metabolism, whereas reducing carbohydrate intake leads to increased fat burning. (For more details on this research, see Lancet 378: 826-847, 2011, featured in the “Research Briefs” section of the September-October 2011 NIH Catalyst). Hall is also collaborating with NIMH investigators to study the effect of dietary changes on brain chemistry.
The MCRU facility, led by clinical director Monica Skarulis and clinical investigator Kong Chen, features three state-of-the-art metabolic suites that are specially designed to accommodate morbidly obese people. Study subjects can be sealed inside the rooms for up to 24 hours, and their metabolic rates can be monitored by measuring oxygen consumption and carbon dioxide production. Room temperature and food intake can be altered and the effect on patient metabolism determined.
Meanwhile, more than 2,000 miles from Bethesda, researchers at NIDDK’s Phoenix Epidemiology and Clinical Research Branch are also tackling obesity. William Knowler leads two longitudinal clinical trials to determine whether long-term intentional weight loss can help prevent or control type 2 diabetes. The notion that weight loss is beneficial for obese people with diabetes is an accepted “fact,” according to Knowler. But, until now, there have been no long-term studies to validate that weight loss prevents diabetes and its resulting complications such as cardiovascular disease and reduced life expectancy. Although the studies will continue through 2014, preliminary analyses have shown that long-term weight loss is possible for diabetic patients, and those at risk for developing diabetes can reduce that risk by 58 percent if they lose weight.

Kong Chen, NIDDK
Clinical Center nurse Cynthia Hahn is demonstrating how blood would be taken from a patient who is in one of the metabolic suites. The “patient” is really clinical investigator Francesco Celi who posed for the photo.
Leslie Baier, Knowler’s colleague in Phoenix, is trying to identify and characterize susceptibility genes for type 2 diabetes and obesity in Pima Indians. Pima Indians have the highest reported prevalence of type 2 diabetes of any population in the world, and many are obese. Baier uses molecular genetic approaches to look in the Pima population at genes already associated with obesity or diabetes in Caucasians, and she is conducting genome-wide searches for new genes associated with obesity and diabetes. She hopes what she learns will help clinicians identify people at risk for these diseases and provide more effective interventions.
With increasing numbers of Americans becoming overweight or obese and the incidence of these conditions also rising worldwide, the obesity research program at NIDDK is helping to shed light on the complex factors involved in this multifaceted disease. The unique facilities at NIH and the diverse nature of the intramural program are allowing investigators to study the problem from multiple angles. A physics degree may not seem a likely weapon in this fight, but NIH is hitting obesity with everything it’s got.
Fat Cells Regulate Metabolism
Haiming Cao may be new to NIH—he came to NHLBI in 2011 as a tenure-track investigator—but he’s no stranger to fat. During his postdoctoral training, he studied the metabolomics of fat and found that palmitoleate, a hormone naturally produced by fat, enhances insulin action and reduces lipid accumulation in the liver. Palmitoleate may protect against diabetes and weight gain.
At NIH, Cao is identifying other fat- and liver-secreted proteins that signal the metabolic state of tissues. He is using proteomics, the study of the entire complement of proteins produced by an organism, to screen potential signaling proteins. So far, his laboratory has identified more than 200 targets. Next, he plans to measure concentrations of target proteins in obese mice under different feeding conditions.
Corresponding changes in the concentration of a target protein could indicate that it modulates the metabolic response of the obese mice. If any of the new target proteins show a metabolic effect, Cao would work with clinical collaborators to measure concentrations of those proteins in serum samples from obese patients.
Cao would also like to generate a transgenic mouse that expresses the target protein, raise an antibody to that protein, and use it to suppress that protein in mice. Successful treatment of mice could indicate a potential therapy for humans.
Parkinson Disease Protein May Regulate Fat Metabolism
One of the NHLBI researchers studying obesity is Michael Sack, chief of the Laboratory of Mitochondrial Biology in Cardiometabolic Syndromes. He may have found a connection between Parkinson disease and obesity. He led a team of NHLBI and NINDS scientists that found that Parkin, an important protein linked with some cases of early-onset Parkinson disease, regulates how cells take up and process dietary fats. Laboratory mice with a defective Parkin gene did not display obvious signs of Parkinson disease, nor did they gain weight even when fed a high-fat diet. The researchers saw a similar pattern in humans when they analyzed blood cells from patients enrolled at the NIH Parkinson Clinic. In lab tests, cells from people with the defective gene for Parkin were less able to absorb fat than were cells from people without the defective gene. (J Clin Invest 121:3701–3712, 2011)
Sack is following up this initial finding with an in-depth clinical study. He plans to collect more data from Parkinson patients and matched control subjects to generate a metabolic profile that would include measures of insulin resistance and body fat.
Sack also plans to collect skin biopsies to obtain fibroblasts from Parkinson patients. The fibroblasts will be made into induced-pluripotent stem cells that can be converted into various cell types. Sack hopes to generate fat cells to further characterize their metabolic findings and dopaminergic neurons to study the neurons that are primarily affected in Parkinson disease.
In collaboration with the NIH Regenerative Medicine Center and the NINDS, Sack will use the dopaminergic neurons to investigate the effect of disrupted lipid metabolism on Parkinson susceptibility. The brain requires a relatively large amount of fat for normal function. Cholesterol and lipid species are needed for neurotransmission and membrane integrity. For mitochondria in particular, lipid content is key to proper function. Disruption of these lipids may make mitochondria less able to handle oxidative and free radical stress. Therefore, impaired mitochondria may contribute to the death of dopaminergic neurons that characterizes Parkinson disease.
Cancer Cells Need Fat to Survive
Tumor cells require large amounts of energy, and one of the primary sources of energy resides in adipose (fat) tissue. Perhaps it was only a matter of time until a molecular target for treating cancer emerged with a distinct role in another highly relevant disease: obesity.

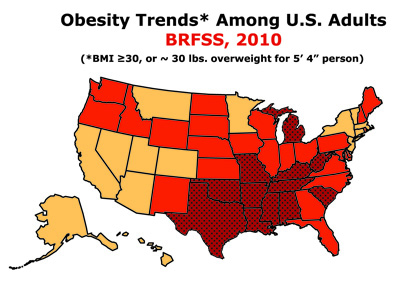
SOURCE: BEHAVIORAL RISK FACTOR SURVEILLANCE SYSTEM CDC
More than one third of adults in the U.S. are obese (with a body mass index of 30 or greater). For an animated map showing obesity prevalence from 1985 through 2010, go to http://www.cdc.gov/obesity/data/trends.html.
In 2009, Morrison and collaborators discovered that the multifunctional molecular scaffolding protein for Ras signaling, kinase suppressor of Ras 2 (KSR2), which is involved in cell growth and differentiation, also interacts with AMPK and is essential for AMPK-dependent regulation of energy metabolism. These findings provided new insight into the roles of these proteins in insulin pathways, demonstrating in mice that the deletion of KSR2 leads to obesity and insulin resistance and that these knockout mice conserve energy and store fat with high efficiency. Deborah K. Morrison, chief of NCI-CCR’s Laboratory of Cell and Developmental Signaling (Frederick, Md.), stumbled upon this discovery a couple of years ago. Her lab focuses on cellular growth mechanisms regulated by the rat sarcoma (Ras) guanine triphosphatase (GTPase) protein and its associated signal transduction pathways, particularly in relation to tumor growth and formation. Energy homeostasis, the body’s balancing act of modulating immediate and long-term energy needs, is achieved through a complex system of regulatory cell-signaling pathways. The enzyme 5' adenosine monophosphate (AMP)–activated protein kinase (AMPK) serves as one of several master regulators by stimulating glycolysis and fatty-acid metabolism as well as by aiding in the insulin-dependent cellular uptake of glucose.
Shortly thereafter, Morrison and co-workers identified yet another family of scaffolding proteins, connector enhancers of KSR (CNK), that plays a similar dual role in both kinase signaling cascades and insulin regulation.
These discoveries came as a surprise. “We did not expect that these particular scaffolding proteins for Ras signaling would be directly involved in these metabolic pathways,” said Morrison. “We expected them to be primarily involved in cell proliferation and cell division.”
The Morrison lab is also designing agents that can target specific domains of KSR2 and CNK1 scaffolding proteins in order to efficiently and selectively inhibit particular functions. “Targeting these scaffolding proteins can be difficult,” said Morrison. “Our goal is to disrupt key protein-protein interactions by targeting specific protein domains without negatively affecting others.” Discovering the novel role of certain scaffolding proteins in the regulation of energy metabolism is certainly a major contribution, not only to cancer research, but also to treating obesity.
Multifaceted Research Program
Understanding the relationship between obesity and its related medical complications—diabetes, heart disease, and other illnesses—is the basis of Jack Yanovski’s research at NICHD. Yanovski, senior investigator and chief of the Section on Growth and Obesity, has devoted his career to understanding the etiology of obesity. In a letter to the New England Journal of Medicine, he noted the need for interventions in early childhood to safely and effectively prevent obesity. He also emphasized the need for treatment to promote and sustain weight loss for obese individuals. (N Engl J Med 364:987-989, 2011).
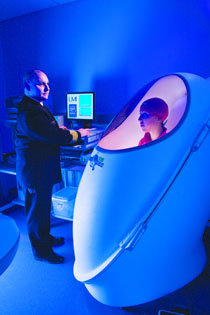
ADDISON GEARY, PENN MEDICINE
As Jack Yanovski monitors the readings, former research associate Laura Wolkoff sits inside the Bod Pod. The machine measures body composition (fat mass and fat-free mass) non-invasively.
Yanovski’s interest in the causes of childhood obesity began during his pediatric endocrinology fellowship at NICHD in 1989. While studying Cushing syndrome, a hormone disorder caused by high concentrations of cortisol in the blood, he observed that most individuals being evaluated for the syndrome had become obese not as a result of problems with adrenal steroid production but for other, unknown, reasons. This early work foreshadowed his research into other rare syndromes associated with hyperphagia (excessive intake of food) and obesity. Today his research elucidates the endophenotypes (different clusters of genetically based behavioral and/or metabolic signs, systems, and/or traits found together) that contribute to childhood obesity.
Recently, Yanovski and coworkers studied the efficacy of metformin (an oral antidiabetic drug that suppresses liver glucose production) pharmacotherapy along with a dietician-administered weight-reduction program in children believed to be at high risk for developing type 2 diabetes. Metformin had a modest but favorable effect on body weight and composition and improved glucose homeostasis in obese, insulin-resistant children. Yanovski’s group also showed that depressive symptoms in children correlate with the progression of insulin resistance. They plan to examine approaches for ameliorating depressive symptoms as a method to improve glucose metabolism.He uses a multifaceted approach to reach an understanding of the genetic antecedents of obesity. His work ranges from studies of molecular mechanisms that distribute the signal provided to the brain by leptin, a hormone that plays a key role in appetite and metabolism, to clinical studies that examine pharmacotherapeutic treatment of obese individuals.
Although Yanovski has made progress in understanding both the genetic and physiological causes of obesity, he emphasizes the need for multidimensional research. We are “never going to be satisfied with what we know today,” he said. “We always need to expand what we know … to help our patients.”
An Epidemiological Approach: Obesity and Aging
Two senior investigators in NIA’s Laboratory of Epidemiology, Demography, and Biometry (LEDB) have taken an epidemiologic approach to studying relationships between obesity and aging. Tamara Harris has organized a multicomponent research program to understand how obesity and obesity-related health conditions contribute to disability and life expectancy in old age. Lenore Launer, LEDB’s acting chief, is exploring how body weight contributes to outcomes of neurologic disease including dementia and Alzheimer disease.
Harris became interested in studying weight in older people because she was intrigued by a paradox: People who are overweight or obese are likely to become disabled in old age; yet often overweight and obese people live longer. She established the Health, Aging, and Body Composition Study (Health ABC), a 14-year study of more than 3,000 older Americans to examine this paradox. Participants in the Health ABC study had dual-energy X-ray absorptiometry and computed tomography scans to measure muscle and adipose tissue. One of the study’s findings was that people who are overweight or obese tend to have more fat in their muscle than lean people. Surprisingly, fat infiltration in muscle was a stronger predictor of disability in old age than was low muscle mass.
In old age, people who have been obese or overweight since earlier in life have more difficulty walking and climbing stairs than do people who gained weight only in old age, according to Harris. She and Launer established the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-Reykjavik) to examine risk factors, including genetic susceptibility and gene-environment interaction, in relation to disease and disability in old age. Midlife measures from the Reykjavik Study will be used to investigate the long-standing effects of obesity on health outcomes in old age.
There is intriguing new evidence that suggests obesity in middle age may increase the risk of developing memory problems and Alzheimer disease in old age, according to Launer. Launer is planning an investigation into the role of body weight on neurological outcomes in the AGES-Reykjavik Study. AGES-Reykjavik has one of the largest databases of brain magnetic resonance imaging (MRI) images in the world in addition to detailed health information on participants from mid-life to old age. Launer hypothesizes that linking data on body weight and weight-related conditions to brain structure, size, and MRI indicators of brain pathology may provide additional insight on the relationship between obesity and neurological outcomes in old age.
The investigator’s research emphasizes the importance of earlier life behaviors and exposures in determining health in old age and suggests that maintaining a healthy body weight throughout adulthood helps to promote healthy aging. They hope their work will help identify key periods in life for intervention and treatment and define weight recommendations for healthy aging.
More Obesity Research
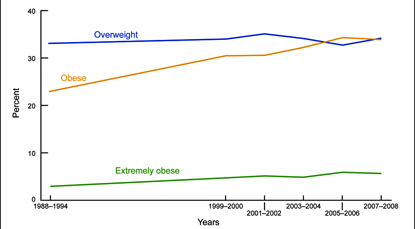
CDC, NATIONAL CENTER FOR HEALTH STATISTICS
Trends in overweight, obesity, and extreme obesity among adults aged 20 years and older: United States, 1998–2008. Overweight is defined as a body mass index (BMI) of 25 or greater but less than 30; obesity is a BMI of 30 or greater; extreme obesity is a BMI of 40 or greater.
There are many other NIH intramural researchers doing obesity-related research in NCI, NHGRI, NHLBI, NIAAA, NICHD, NIDA, NIDDK, NIEHS, and other institutes and centers. The subjects of NIH obesity studies range from the molecular link between metabolic imbalance and breast cancer risk; to metabolic differences in people’s reactions to cold temperatures; to sleep deprivation and weight gain; to food as addiction; to metabolic effects of non-nutritive sweeteners; to the rise in nonalcoholic fatty liver disease, which is linked to obesity; to imaging studies that identify patterns in weight gain and progression in obesity-related diseases; to the relationship between the increase in the food supply and obesity; to environmental chemicals and weight gain; and more.
HBO SERIES ON OBESITY TO DEBUT IN MAY: NIH Researchers Played a Big Role
The HBO series on the obesity epidemic, “The Weight of the Nation,” will air in May and help launch one of the most far-reaching public health campaigns on the epidemic to date. To create the series, HBO partnered with NIH, the Institute of Medicine (IOM), Centers for Disease Control and Prevention (CDC), the Michael and Susan Dell Foundation, and Kaiser Permanente. Several NIH researchers were interviewed, but at press time, it is not known whether they will appear in the program.
The series comprises four documentary films, a three-part HBO Family series, 14 bonus shorts, a social media campaign, a book published by St. Martin’s Press, and a nationwide community-based outreach campaign to support the initiative.
The four-documentary series debuts Monday, May 14, on HBO with two films airing back-to-back that night and two more the next night. The three-part HBO Family series debuts Wednesday, May 16.
“If we don’t succeed in turning this epidemic around, we are going to face, for the first time in our history, a situation where our children are going to live shorter lives than we do,” said NIH Director Francis S. Collins in the press release announcing the program.
This page was last updated on Monday, May 2, 2022
