A Lifetime Exploring the Kingdom of Fungi
IRP’s June Kwon-Chung Elected to National Academy of Sciences for Advances in Mycology

Dr. June Kwon-Chung was elected to the National Academy of Sciences in April 2024 for her discoveries about both benign and disease-causing fungi.
Even as a little girl, NIH Distinguished Investigator June Kwon-Chung, Ph.D., knew she would be a scientist. Seven decades later, she has been elected to the National Academy of Sciences (NAS) for her groundbreaking research on fungal diseases.
“My dream has always been to become a research scientist in the field of biology,” Dr. Kwon-Chung says. “This goes way back to when I was barely out of the toddler stage.”
As a child growing up in Korea before it split into two separate countries, Dr. Kwon-Chung felt blessed to have parents and teachers who nurtured her interests early. Her participation in her junior high school’s biology club led her to focus on the study of bacteria as a student at the Ewha Womans University in Seoul, Korea, even though wartime disruptions made scientific study challenging. In 1961, she won a year-long Fulbright Smith-Mundt scholarship to study in the U.S. and chose to attend the University of Wisconsin, where she specialized in mycology, the study of fungi.
“I took an introductory mycology course in my first year there and the instructor made everything so fascinating,” Dr. Kwon-Chung remembers. “I was stunned by the fungal mega-diversity in size and role in the ecosystem, as well as their medical and economic importance.”

The truffles that we pay a pretty penny for in restaurants are actually a type of fungus, but not all fungi have such pleasant effects when they enter our bodies.
Mold, yeast, mushrooms — the world of fungi is diverse, and to most of us, somewhat mysterious. The fungal kingdom includes more than 150,000 described species, which can live in virtually any environment and range in size from single-celled organisms to fungal networks that can stretch across an area larger than a football field. They can be exotic delicacies in fine restaurants, necessary ingredients for rising bread and fermenting soy, and annoying or dangerous pests infecting our bodies and homes. What’s more, without their help breaking down organic materials, Earth’s surface would be buried under piles of dead animals and plants.
As much good as fungi can do, certain fungi can cause disease when they take parasitic hold in the body. Many can be easily disposed of by a healthy immune system, while others cause irritating ailments like yeast infections and athletes’ foot. Fungal infections can even be deadly, especially to people with weakened immune systems. These are the ones Dr. Kwon-Chung has spent the last six decades investigating.
“There are thousands and thousands of fungal elements we are exposed to, but where they live in the environment is so different from the host body,” Dr. Kwon-Chung says. “It is important to understand the characteristics of these pathogens to effectively combat the diseases they cause. We have to understand the enemy we are facing, such as their virulence factors, which enable environmental fungi to successfully multiply in host organs that drastically differ from their natural habitat.”
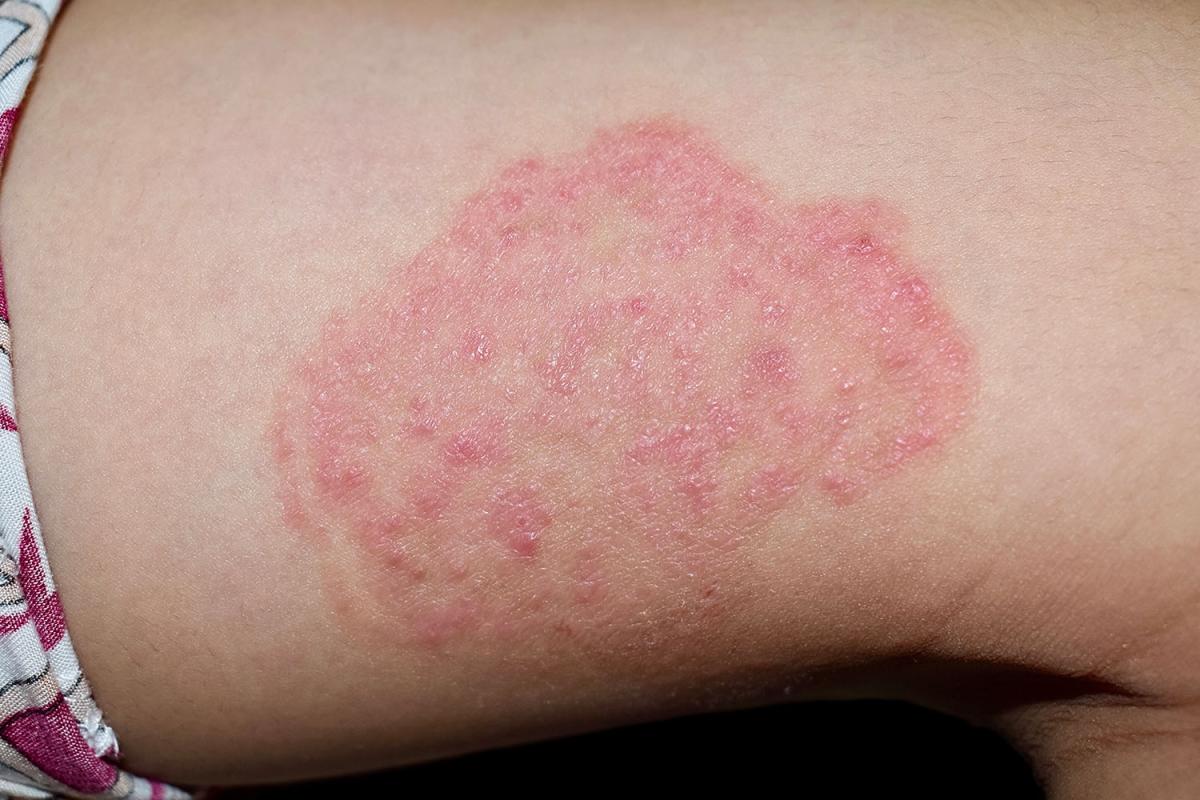
Fungi can cause ailments ranging from irritating rashes to deadly brain ailments.
As a graduate student at the University of Wisconsin, Dr. Kwon-Chung discovered the first ‘heterothallic’ species of the Aspergillus fungus, a term that means the fungus must mate with compatible strains of the opposite mating type in order to reproduce sexually. This set up both her interest in the lifecycles of disease-causing fungi and her reputation as a rising star. In the decade after she arrived at NIH in 1966 as a Fogarty International Fellow, she discovered additional examples of heterothallic fungi and worked out the mechanisms through which they exchange genetic material during mating. Among the heterothallic fungi she identified were Histoplasma capsulatum and Cryptococcus neoformans, which can both cause serious fungal diseases. Dr. Kwon-Chung ended up focusing on Cryptococcus and named its two mating types — akin to other organisms’ two genders — ‘mating type alpha’ and ‘mating type a’.
“Once I identified the two mating types of Cryptococcus, a whole different field of work opened up,” she says. “Now I could ask epidemiological questions like how common is mating type alpha compared to mating type a in nature or how often is it the cause of a fatal infection.”
Of course, as a scientist at an institution dedicated to the mission of improving health, Dr. Kwon-Chung and her research team have focused not only on the life cycles of disease-causing fungi like Aspergillus and Cryptococcus, but also the factors that influence their ability to infect people and resist treatments. Aspergillus is a mold found on decaying leaves and compost heaps as well as in air conditioners, while Cryptococcus is found in pigeon droppings, soil contaminated with those droppings, and on the bark of certain trees. These fungi are constantly releasing reproductive material into the air in the form of dehydrated yeast particles or spores, which are usually harmless but can be extremely dangerous for certain individuals.

The disease-causing fungus known as Cryptococcus spreads far and wide thanks to its ability to hitch a ride in the droppings of migrating pigeons.
“We humans are exposed to these fungi regularly, and if healthy individuals inhale their reproductive material, our immune system takes care of them,” Dr. Kwon-Chung says. “But if the immune system is weakened, they begin multiplying in the lungs and spread through the bloodstream to other organs, such as the brain in the case of Cryptococcus.”
In fact, 19 percent of people with AIDS die from Cryptococcus infections. Such conditions are so prevalent among HIV/AIDS, patients that cryptococcosis is considered one of the diagnostic hallmarks of AIDS. In the lungs, the fungus causes serious pneumonia and eventually travels to the brain, where it causes meningoencephalitis — inflammation of brain tissue and the membrane surrounding the brain and spinal cord. Untreated, cryptococcal meningoencephalitis is 100 percent fatal; even with the most advanced treatments, about 30 percent of patients die.
Dr. Kwon-Chung’s discoveries about the sexual lifecycle of Cryptococcus not only placed it in the correct position within the fungal classification system, but also helped reshuffle part of the fungal family tree. At the time of her findings, fungi were classified based on their characteristic form of sexual reproduction. Once it became clear that the asexual species of fungi could have sexual states, scientists stopped classifying them based on their form of asexual reproduction. Her new discoveries also meant it would be possible to cross-mate generations of fungi to study their genetic traits, including factors that help them infect people. This ability was much needed in the days before DNA sequencing.

The fungus Cryptococcus neoformans, pictured here, can cause brain inflammation, known as meningoencephalitis.
More recently, Dr. Kwon-Chung and her colleagues discovered an unusual form of drug resistance that is entirely different from the usual evolutionary battle between our bodies and the fungi that infect us. They found that cryptococcosis-causing fungi are able to undergo a process called heteroresistance, in which whole chromosomes that harbor genes relevant for resistance can duplicate when exposed to anti-fungal drugs. Once the drug pressure is off, however, the fungi lose these extra copies of their chromosomes and the accompanying drug resistance.
“We found this phenomenon about a decade ago, but we are still working on it,” she says. “Because there is no precedent for the phenomenon, we still have a ways to go to completely understand the mechanism of chromosome duplication under drug stress.”
Those kinds of bizarre characteristics are what have kept Dr. Kwon-Chung passionate about her research for decades. Now, at age 90, she has no plans to stop any time soon. With the help of her IRP colleagues, she hopes to reveal even more of the eccentricities of the fungal kingdom.
“I enjoy working at NIH because of the freedom to choose subjects that I'm interested in, and I get sufficient support to pursue them,” she says. “Also, you can readily find collaborators with the expertise you need. I believe that one reason I have sustained more than half a century of benchwork at NIH is partly due to the support of these colleagues. How can it not be a good place to work?”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Monday, July 29, 2024
