Researchers Display Discoveries at Annual Research Festival
A Showcase of Intramural Science Inspires
BY BRANDON LEVY, IRP
No single event captures the incredible breadth of intramural research as effectively as the annual NIH Research Festival. In fact, the event is so jam-packed that it stretches over multiple days, running this year from September 23 to 25. The first day of the 2024 Research Festival kicked off with a series of poster sessions in which scientists from across NIH showcased the innovative science they have been working on, demonstrating research on subjects such as how cooking affects the brain, the impact of vaping on lung health, and 3D models for studying pregnancy complications. Read on to dive deeper into a sampling of the more than 400 research projects presented at this year’s celebration of science.
Siobhan Lawler: Bringing neuroscience into the kitchen
CREDIT: BRANDON LEVY
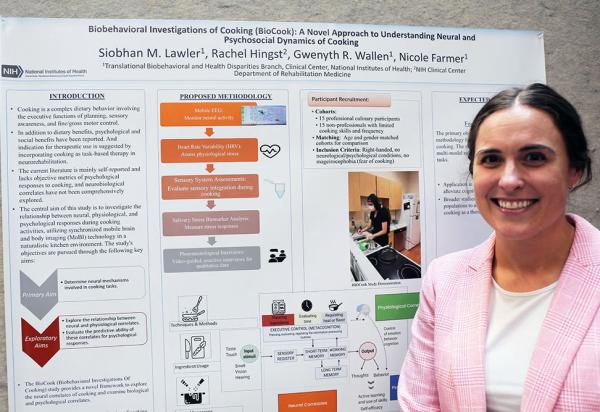
Siobhan Lawler presented a research poster titled “Biobehavioral Investigations of Cooking (BioCook): A Novel Approach to Understanding the Neural and Psychosocial Dynamics of Cooking.”
Siobhan Lawler landed her first position at NIH through the Graduate Summer Opportunity to Advance Research (G-SOAR) program, which provides graduate students interested in pursuing a doctorate with the opportunity to spend a summer working in an intramural NIH lab. Now, after completing her doctorate and a postdoctoral fellowship in another NIH research group, she is in the midst of a second postdoctoral fellowship with Nicole Farmer, who studies how cooking might help improve people’s mental health and reduce their risks of developing certain illnesses.
The particular study Lawler is working on, called BioCook, aims to determine how cooking affects bodies and minds.
“While cooking has been associated with psychological, social, and dietary benefits, most existing research relies on self-reported data and lacks rigorous scientific investigation with objective measurements,” Lawler explained. “Therefore, the BioCook study aims to fill this gap by employing a multimodal approach, including [electroencephalography], heart rate variability monitoring, sensory system assessments, repeated salivary stress biomarker analysis, and in-depth video-guided interviews conducted after the cooking task.”
Having sampled life in several IRP labs, Lawler says working with her colleagues in Farmer’s lab has been particularly rewarding. Lawler says Farmer is deeply invested in her growth and incredibly giving of her time and guidance.
Madison Hernandez: Tackling Tay-Sachs with gene editing
CREDIT: BRANDON LEVY
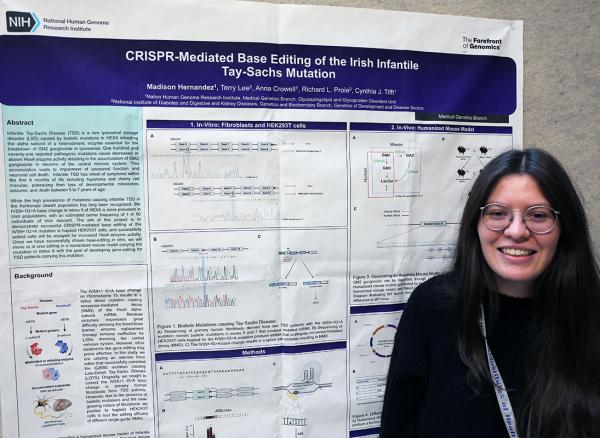
Madison Hernandez presented a research poster titled “CRISPR-mediated Base Editing of the Irish Infantile Tay-Sachs Mutation.”
Although Madison Hernandez was able to participate in research as an undergraduate at the University of Texas of the Permian Basin (Odessa), she says that she was unable to pursue her niche interests until she landed the opportunity to work as a postbaccalaureate research fellow under the tutelage of NHGRI Senior Clinician Cynthia Tifft. With Tifft’s help, Hernandez was able to conduct research she finds personally meaningful to her.
“I was born with a nonprogressive neurological disorder that affects my movement, which ultimately inspired my interest in investigating rare neurodegenerative diseases,” Hernandez said. “I grew interested in gene therapy because the idea of correcting a disease at its source fascinated me. When I applied for the NIH postbac program, I contacted Tifft almost immediately because her interests aligned so closely with mine.”
Although Tifft’s lab studies several rare neurological conditions, the one Hernandez has been focused on for the past year and a half is infantile Tay-Sachs disease, which is caused by mutations in both copies of the HEXA gene. Without a functional HEXA gene, an enzyme called GM2 ganglioside accumulates in neurons with toxic effects, leading to loss of motor coordination, seizures, and eventually death.
To combat this tragic disease that kills so early in life, Hernandez is trying to figure out how to use the CRISPR-Cas9 gene-editing technique to correct the HEXA mutation. However, unlike traditional CRISPR-Cas9 gene editing, which breaks apart both strands of the DNA molecule before stitching them back together, Hernandez is pursuing an alternate approach called “base editing,” which can correct a single DNA base pair without causing a double-strand break.
“Our findings would provide a proof of concept on base editing for this Tay-Sachs mutation, which could later be used to develop a more efficient gene therapy for patients carrying this specific mutation,” Hernandez explained.
Tommy Jones: Designing a device to enable cutting-edge cancer research
CREDIT: BRANDON LEVY
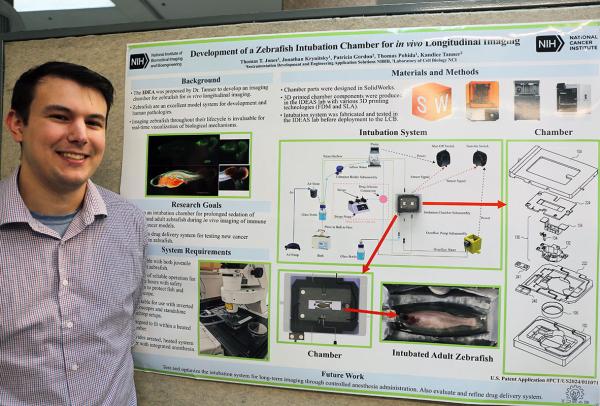
Tommy Jones presented a research poster titled “Development of a Zebrafish Intubation Chamber for In Vitro Longitudinal Imaging.”
Tommy Jones designs and builds new contraptions in NIBIB’s Instrumentation Development and Engineering Application Solutions (IDEAS) section.
“The IDEAS section provides engineering expertise to NIH researchers, specializing in the development of first-of-a-kind instrumentation design, software development, in-house fabrication of prototypes, and overall collaborative validation in labs and clinical settings,” Jones explained.
At this year’s Research Festival, Jones showed off the design he and his IDEAS colleagues created for a zebrafish (Danio rerio) sedation chamber that will help scientists in the lab of NCI-CCR Senior Investigator Kandice Tanner use that model organism to learn about why certain types of cancer spread to specific parts of the body. The chamber can keep zebrafish sedated for the multiple hours it takes for Tanner’s team to take images of the fish’s inner workings, and it can work with the multiple types of advanced microscopes Tanner’s team uses. The research also requires that the zebrafish remain alive so that the same fish can be examined in the same manner, repeatedly.
“Typically, zebrafish imaging is done in short intervals to ensure the fish’s survival,” Jones said. “This design enables longer, continuous imaging sessions while preserving the health of the fish. We also designed it to have the ability to look at different developmental stages from younger fish to adults, as all the previous devices were for adults only.”
Malique Jones: Making muscle cells in a Petri dish

CREDIT: BRANDON LEVY
Malique Jones presented a research poster titled “Generation of Human iPSC-derived Skeletal Myotubes to Evaluate DOK7 and COLQ Targeted Therapeutics.”
Malique Jones is leveraging her experience in the field of muscle biology to develop a sort of “muscle-in-a-dish” model that could help researchers test treatments for a particular set of rare neuromuscular disorders.
Jones is a postdoctoral fellow at NCATS working with Catherine Chen in the Therapeutic Development Branch of the Division of Preclinical Innovation. The research team is nurturing induced pluripotent stem cells (iPSCs), which can develop into any other kind of cell found in the body, in a manner that coaxes them to become long cells found in our muscles called myotubes.
“This effort is part of the NCATS Platform Vector Gene Therapy program and establishment of an efficient two-dimensional skeletal myotube model that will provide an excellent resource for advancing preclinical testing and gene therapy development for treatment of rare diseases,” Jones explained.
Ethan Greenstein: Addressing autism’s auditory challenges with brain stimulation
CREDIT: BRANDON LEVY
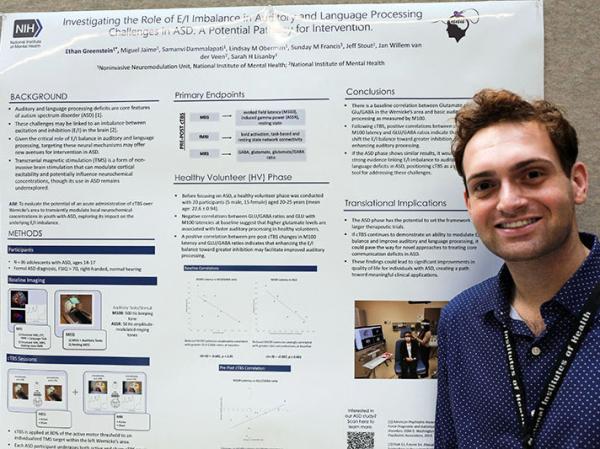
Ethan Greenstein presented a research poster titled “Investigating the Role of E/I Imbalance in Auditory and Language Processing Challenges in ASD: A Potential Pathway for Intervention.”
It makes perfect sense that an organ as vital as the brain is encased in our thick, hard skulls to shield it from the outside world. Unfortunately, this makes it all the more challenging to treat psychiatric conditions by influencing the brain’s electrical firing.
Ethan Greenstein applies a technique called repetitive transcranial magnetic stimulation (rTMS), which uses magnetic fields to alter electrical activity in the brain. Greenstein works with Sarah Lisanby at NIMH to investigate whether a particular form of TMS might improve how the brains of people with autism spectrum disorders (ASD) process information about sound and spoken language.
“When I learned about the cutting-edge research being done in Lisanby’s lab, especially in the area of noninvasive brain stimulation to more broadly treat mental health conditions, I knew I wanted to be part of the team,” he said.
The study, known as DECIBELS, uses rTMS to shift the balance between excitatory signals in the brain that activate neurons and inhibitory signals that make neurons less likely to fire. Scientists think an imbalance between these two types of signals is what underlies the challenges experienced by people with ASD. Their work has already revealed that rTMS treatment can change the ratios of excitatory and inhibitory chemicals in the brains of cognitively typical individuals, and this change was associated with their responding more quickly to sounds. Now, they hope to see similar results when applying the same approach to individuals with ASD.
“This research is important because social communication challenges are a core feature of ASD, and current interventions do not specifically target the underlying neurochemical imbalances that may contribute to these difficulties,” Greenstein explained, adding that exploring noninvasive brain stimulation as a potential intervention could open new pathways for addressing key ASD-associated challenges.
Read more about each researcher and the work they do at NIH—including fun facts about each of these interesting individuals—on the “I Am Intramural Blog.”
Brandon Levy is a health communications specialist for the NIH’s Intramural Research Program. When he is not hunched over a computer keyboard, he enjoys singing in the NIH acapella group “Nerds In Harmony,” as well as reading, honing his skills as an amateur chef, and over-obsessing about college basketball.
This page was last updated on Tuesday, November 5, 2024
