Research Briefs
Read about scientific advances and discoveries by NIH intramural scientists: an endocrine disrupting chemical found in a common fragrance; infections linked to neurodegeneration; an antibody that protects against Nipah virus; how diet and obesity changes the brain; fotonovelas used to educate patients about cancer risk; and how pregnancy alters the brain in dynamic ways.
NCATS, NIEHS: COMMON FRAGRANCE LINKED TO EARLY PUBERTY IN GIRLS
A research team led by NCATS and NIEHS used the Tox21 compound library to find chemicals that could activate key endocrine targets in the hypothalamus and might be driving the rising trend toward premature puberty in girls. Read more here. (NIH authors: S. Yang, L. Zhang, J. Travers, R. Huang, V.M. Jovanovic, R. Veeramachaneni, S. Sakamuru, C.A. Tristan, C. Klumpp-Thomas, K.L. Witt, A. Simeonov, N.D. Shaw, and M. Xia, PMID: 39254333)
Learn more about the potential environmental influences on early puberty from Natalie Shaw, principal investigator, NIEHS Pediatric Neuroendocrinology Group.
NIA: INFECTIONS AND IMMUNE-SPECIFIC PROTEINS MAY INCREASE DEMENTIA RISK AND BRAIN ATROPHY
CREDIT: MICHAEL DUGGAN, NIA
Infections can promote the development of dementia and other negative neurocognitive outcomes (lower arrow) and can directly lead to brain atrophy and neurodegeneration (upper arrows).
NIA research suggests infections and varying concentrations of immune-specific proteins may contribute to increased dementia risk and brain volume loss in older adults. Although other scientists have found a link between infections and cognitive decline, this study identified the proteins involved and used a genetics tool to support the protein connection between infection, dementia risk, and neurodegeneration.
The research team was led by NIA tenure-track investigator Keenan Walker and NIA postdoctoral fellow Michael Duggan. They examined medical records of participants in the Baltimore Longitudinal Study of Aging (BLSA) looking for verification of infections from influenza, herpes, and other viruses as well as bacterial and fungal infections in the upper respiratory system, urinary tract, and skin. After separating plasma from approximately 1,200 blood samples obtained via the BLSA, team members measured more than 7,000 proteins in plasma. They then used magnetic resonance imaging to conduct brain scans on nearly 1,000 BLSA participants. The scientists processed the data using a technique called Mendelian randomization, an analysis that uses statistical genetics to estimate a cause for a particular outcome.
The scientists identified 35 immune-specific proteins that correlated with infection history and predicted patterns of brain atrophy that were specific to infections. Further investigation revealed that individuals who had infections on average 16 years earlier still had higher concentrations of damaging proteins and lower concentrations of neuroprotective proteins circulating in their bodies. The team theorized that genetic variability could provide the backdrop for infection susceptibility and altered immune protein regulation, both of which can contribute to increased dementia risk and brain atrophy. (NIH authors: M.R. Duggan, Z. Peng, J. Candia, T. Tanaka, C.M. Joynes, C.X. Alvarado, M.A. Nalls, J. Cordon, G.N. Daya, Y. An, L. Ferrucci, and K.A. Walker, PMID: 39143319)
[BY ROBIN ARNETTE, NIA]

Read more about Walker’s work on the “I Am Intramural” blog.
NIAID: TREATMENT DEMONSTRATES PROTECTIVE EFFICACY AGAINST NIPAH VIRUS
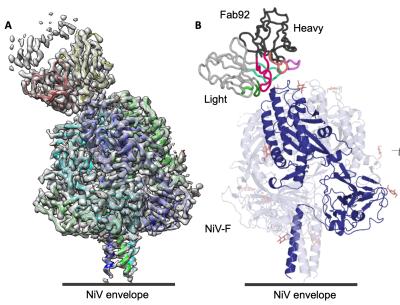
CREDIT: NIAID, JOURNAL OF VIROLOGY
The cryo-EM structure of the Nipah virus’s NiV-F–Fab92 complex revealed where the mAb92 antibody binds to NiV-F, which could inform future therapeutics or vaccines.
Emerging cases of the lethal Nipah virus (NiV) in South Asia have prompted the World Health Organization to classify NiV as a priority pathogen. NiV is a highly pathogenic bat-borne virus that triggers severe neurological and respiratory diseases in humans and other animals. According to the CDC, the mortality rate in humans is 40%–70% and treatment is limited to supportive care. Now, an international team led by NIAID researchers have found that a vaccination-derived neutralizing monoclonal antibody, mAb92, provides complete protection against NiV disease in hamsters (Mesocricetus auratus).
NiV is shed via the urine from infected large fruit bats, also called flying foxes (Pteropus genus). Humans can become infected through direct contact with fruit or sap contaminated with the virus, through exposure from infected animals such as pigs, or from contact with an infected human.
In a study led by Vincent Munster, chief of the Virus Ecology Section at NIAID’s Rocky Mountain Labs, researchers tested the ability of mAb92 to target the NiV fusion glycoprotein (NiV-F), and found that interfering with NiV-F prevents the virus from attaching to and entering cells. Cryo-electron microscopy, or cryo-EM, analysis revealed precisely where mAb92 binds to NiV-F, thus providing a path for the development of therapeutics or vaccines.
The authors noted that in work by other research teams another monoclonal antibody, mAb102.4, offered protection against NiV and a similar virus, Hendra, in the African green monkey (Chlorocebus aethiops) and was safe in a phase 1 clinical trial in Australia. (NIH authors: V.A. Avanzato, T. Bushmaker, C.K. Yinda, K. Meade-White, R. Rosenke, T. Thomas, N. van Doremalen, G. Saturday, and V.J. Munster, PMID: 39240113)
[BY HÉCTOR CANCEL–ASENCIO, NINDS]
NIAAA, NINR, NIMH, NIDCD: DIET-INDUCED OBESITY LEADS TO GENETIC DIFFERENCES IN BRAIN REGIONS ASSOCIATED WITH EATING BEHAVIOR
A team of researchers from NIAAA, NINR, NIMH, and NIDCD’s newly established NIH National Smell and Taste Center, along with external collaborators, have identified several genetic changes in the brains of obese mice associated with diet.
Obesity, characterized by an excessive accumulation of body fat, is linked to a range of serious health issues. One major factor contributing to obesity is the overconsumption of high-fat foods, which are often rich in flavor and calories. The chemical stimuli from these foods can be particularly rewarding, leading individuals to eat more than necessary and, consequently, gain weight.
Recognizing this shift in eating behavior, NIH researchers suspected that genetic alterations might occur in the obese brain, reinforcing patterns of overconsumption. To explore this idea, they examined gene expression in obese mice fed a high-fat diet and compared the results with expression in controls.
The study focused on two critical brain regions—the striatum and the olfactory bulb—that play a role in our eating behavior. In obese mice, 274 differentially expressed genes were identified in the striatum, along with 11 in the olfactory bulb. These genes were associated with inflammation and immune-related pathways, mitochondrial dysfunction, and reward.
“This [result] means that our diet is associated with gene expression differences in our brain,” said Rosario Jaime-Lara, first author of the study, who was previously an NIH fellow at NIAAA and is now an assistant professor at the University of California at Los Angeles. “It also suggests that our diet may impact inflammation, the immune system, and reward pathways in the brain.”
As obesity and related diseases increase worldwide, the authors note the importance of understanding modifiable risk factors, such as diet, and how they affect our bodies. “We hope that by understanding the underlying mechanisms and risk factors, we can better manage and prevent obesity and its comorbidities,” Jaime-Lara said. (NIH authors: R.B. Jaime-Lara, C. Colina-Prisco, M. De Jesus Vega, S. Williams, T. Usdin, A. Kinkead, B. Brooks, Y. Wang, A.T. Franks, and P.V. Joseph, PMID: 39273278)
[BY MEAGAN MARKS, NIAAA]
NCI: FOTONOVELAS ARE PICTURE-PERFECT FOR TAILORED EDUCATION ON GENETIC CANCER RISK

CREDIT: NCI
Fotonovelas can be used as a culturally tailored tool to increase knowledge of genetic counseling and testing among Latina women at risk of hereditary breast and ovarian cancer.
Latinas are more likely than non-Latina Whites to be diagnosed with aggressive subtypes of advanced-stage breast cancer. Genetic cancer risk assessment is recommended for women at risk of hereditary breast and ovarian cancer (HBOC) and includes genetic counseling and testing (GCT). However, compared with non-Latina Whites, Latinas are less likely to receive GCT because of systemic and patient-level barriers.
Fotonovelas, a well-accepted story-telling medium in Hispanic cultures, can be effective for communicating health education messages, an NIH-led study has found. A bit like a comic book but with real photographs, a fotonovela might include a short narrative, speech balloons, simple text, or engaging visual elements. Hypothesizing that fotonovelas, through storytelling, can help with learning and recalling information because of the reader’s connection to a story, NCI researchers and their extramural colleagues developed and tested fotonovelas to increase awareness of GCT among Latinas at risk of HBOC. Content was drawn from an existing culturally targeted narrative video focused on improving GCT among the study’s population.
The research team interviewed cancer patients and their relatives and found that the use of fotonovelas had several positive health effects. They reported an increased GCT self-rated knowledge from 50% to 60% in patients and from 63% to 100% in relatives, and an increased willingness to talk about cancer with family from 70% to 100% in patients and from 38% to 75% in relatives.
The team also identified and discussed six themes with participants and health workers, including perceived barriers to GCT. They concluded that fotonovelas could be used as educational tools to increase GCT awareness and cancer conversations among Latino families at risk of HBOC. They noted that, although their findings need to be replicated in a larger study, future research could explore ways to use fotonovelas for disseminating information about and access to GCT and cancer more broadly. (NIH authors: R. Barajas, M. Rotunno, and E. Gillanders, PMID: 39240499)
[SEPPIDEH SAMI, CC]
NIMH: THE EFFECTS OF PREGNANCY ON THE BRAIN
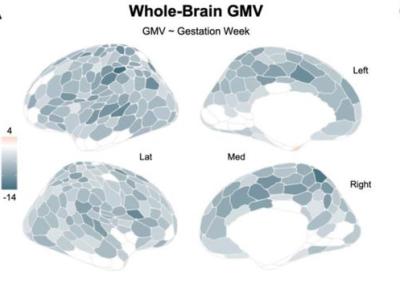
CREDIT: NATURE NEUROSCIENCE
A research team studied 26 magnetic resonance imaging (MRI) brain scans throughout pregnancy and found a widespread reduction in cortical gray matter volume. Darker colors represent regions of greater volume loss in the image above, which reflects analysis of MRI data.
Neural changes that unfold in the maternal brain throughout gestation have not been well studied, but now a guide might be on the way: A team of researchers from the University of California at Irvine and Santa Barbara collaborated with NIMH scientists to map a human brain across pregnancy, which may provide better understanding of postpartum depression and similar neurological concerns.
Using precision magnetic resonance imaging, researchers mapped the brain of a healthy 38-year-old woman beginning three weeks preconception through two years postpartum. Corresponding author and neuroscientist Elizabeth Chrastil of the University of California at Irvine was the woman whose pregnancy—and brain—was followed in this study.
The scientists found a widespread reduction in cortical gray matter volume (GMV) in nearly 80% of brain regions with an average reduction of around 4%, with just a small rebound postpartum. They also found transient increases in white matter microstructural integrity, which is a measure of the health and quality of the connections between brain regions. “This increase peaked around the second and third trimester and returned to baseline postpartum, so a standard before versus after pregnancy study would have missed this finding,” said Chrastil. “This is the first time we have been able to see these types of dynamic changes occurring during pregnancy.”
On average, change in cortical GMV was nearly three times that of control samples. Joshua Faskowitz and Daniel Handwerker in NIMH’s Section on Functional Imaging Methods provided expertise in imaging and analysis in the study which was funded in part by NIA.
Although an “N-of-1” study, these findings indicate that during pregnancy the human brain exhibits remarkable changes. Currently, clinicians lack the tools needed for detection and treatment of neurological disorders that co-occur or worsen with pregnancy. Precision imaging could aid doctors in assessing an individual’s risk for certain conditions during pregnancy as well as direct the creation of new therapeutics for neurological disorders. (NIH authors: J. Faskowitz and D.A. Handwerker, PMID: 39284962)
[BY MELANIE BARKSDALE, NIAID]
This page was last updated on Tuesday, November 5, 2024
