News You Can Use: Technology Transfer
Matching Researchers with Industry to Help Get NIH Inventions to Patients
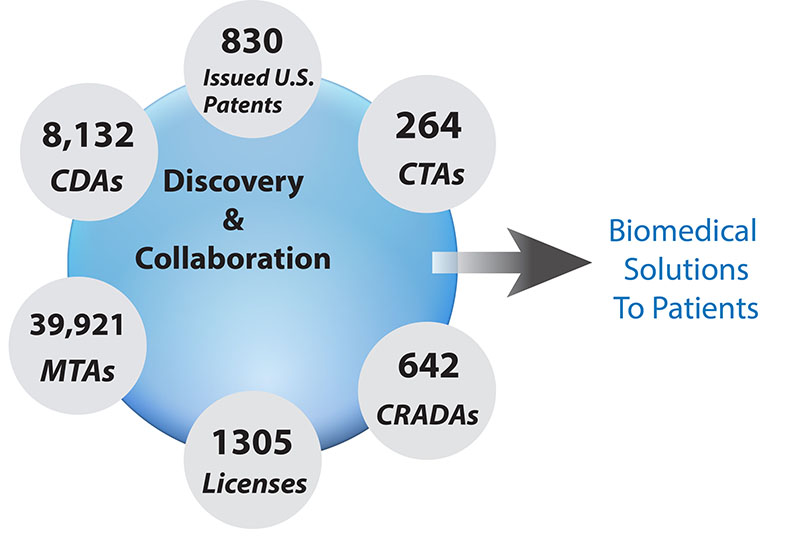
CREDIT: TECH TRANSFER CENTER, NCI
NCI Technology Transfer Center (TTC) metrics for FY2007–18. The NCI TTC handles technology transfer for NCI research labs as well as for labs from nine other NIH ICs. Key: CDA (confidential disclosure agreement); CRADA (cooperative research and development agreement); CTA, (clinical trial agreement); MTA (material transfer agreement).
In September 2016, M was diagnosed with metastatic Merkel-cell carcinoma (MCC), a rare and aggressive form of skin cancer. At the time of his diagnosis, patients with MCC were usually offered standard treatments that included surgery, radiation therapy, and/or chemotherapy. But M’s doctors recommended that he participate in a clinical trial for the immunotherapy drug avelumab. M is now in remission and in March 2017, due in part to the success of that clinical trial, avelumab became the first FDA-approved treatment for MCC.
That drug was brought to market thanks to collaborations between NIH investigators and industry partners. Avelumab, an antibody that targets programmed death–ligand 1, was developed for clinical use by EMD Serono Inc. The company collaborated with National Cancer Institute (NCI) scientists, who performed the preclinical studies and first-in-human clinical trials (conducted at the NIH Clinical Center). The preclinical research showed that avelumab allows T cells to efficiently kill a variety of tumor cells including MCC. The team’s multicenter clinical phase 2 trial—funded by NCI, Merck KGaA of Darmstadt, Germany, and Pfizer—established safety and pharmacokinetic data on the drug and successfully demonstrated the use of avelumab in people with MCC. It is one of three drugs that received FDA approval in the past two years as a result of successful collaborations between investigators in the NCI Center for Cancer Research (NCI-CCR) and commercial partners.
Axicabtagene ciloleucel, another FDA-approved immunotherapy drug that resulted from a successful NIH-industry collaboration, is used to treat adults with relapsed or treatment-resistant large B-cell lymphomas. A team led by NCI-CCR’s Steven Rosenberg developed a technology that led to the drug, which is a CD19-targeted CAR T-cell immunotherapy. After promising early-phase clinical trials conducted at NCI, the drug was licensed to Kite Pharmaceuticals for further development and commercialization and received FDA approval in October 2017.
And, in September 2018, the FDA approved moxetumomab pasudotox, a bacterial toxin–based drug, for the treatment of some patients with hairy cell leukemia (HCL). The antibody moxetumomab was originally generated by Ira Pastan and colleagues at NCI and later licensed to MedImmune/AstraZeneca for clinical development. The FDA approval makes moxetumomab, marketed as Lumoxiti, the first treatment approved for patients with HCL who have already undergone at least two lines of standard treatments.
Commercial Partners
“If you look through the success stories [of] the products that have come out of the intramural program, most if not all of them involve a commercial partner,” said Tom Misteli, the scientific director of NCI-CCR.
NIH does not perform product development or commercialization. And unlike their counterparts at universities, NIH investigators cannot spin out a company around an invention. So it’s essential that there be a way for intramural ideas and technology to be transferred to industry partners.
Working with Your Tech Transfer Office
Tech-transfer coordinators and offices throughout NIH facilitate these collaborations by helping researchers choose from an assortment of agreements—including material-transfer agreements, collaborative agreements, licensing agreements, clinical-trial agreements, and cooperative research and development agreement (CRADAs)—and by managing licenses and patents.
The offices can also help investigators develop translational strategies and find commercial partners. For example, NCI’s Technology Transfer Center (TTC) acts as an “NIH version of Match.com” by advertising NIH technologies to the extramural community and working to find the relevant industry partners to develop those ideas, explained Michael Salgaller, supervisor of the Invention Development and Marketing Unit in NCI’s TTC.
Yet many investigators aren’t aware of all that tech-transfer offices can do for them. “We meet investigators who have been here for years who have no idea how their tech-transfer office can help their ideas be commercialized,” said Salgaller.
Every institute and center (IC) has a technology-transfer coordinator and access to a technology-transfer office. Some ICs have their own tech-transfer offices, whereas others are served by bigger ICs. For example, the National Human Genome Research Institute has its own tech-transfer office and so does the National Institute of Allergy and Infectious Diseases. NCI’s TTC, which is the largest tech-transfer center at NIH, serves nine other ICs; the National Heart, Lung, and Blood Institute serves six others.
How Partnerships Begin
Partnerships between labs at NIH and commercial entities usually begin in one of two ways. In one common scenario, a company may reach out to an investigator after reading their paper(s) or hearing a talk at a scientific meeting. For example, several companies approached NCI investigator Mitchell Ho after seeing posters presented by his postdocs during the 2018 American Association for Cancer Research meeting in Chicago. Ho’s lab develops antibody-engineering technologies that target and validate tumor antigens in solid tumors. He now collaborates with several commercial partners through three CRADAs and three licenses (with other agreements in the works). Collaborating with companies “helps our basic research move to the next step,” he said. Thanks to his commercial partnerships, antibodies to target two cell-surface proteins, glypican-3 (GPC3) on liver cancer cells and GPC2 on neuroblastoma cells, will likely begin in clinical trials this year.
Alternatively, an investigator may need to reach out to their tech-transfer office before talking to a company about their ideas. This is certainly the case if they develop something that may be patentable. Once something is introduced to the public, whether by talk or publication, it “starts a [patent] clock ticking,” said Mark Rohrbaugh, NIH Special Advisor for Technology Transfer. He explained that a patent must be filed in the United States within a year, but foreign rights are lost upon publication. “Check with your tech-transfer office before presenting your invention publicly.”
Challenges and Resources
Many of the ideas and inventions that come out of NIH labs are very early in product development and sometimes too risky for a commercial partner to be interested. But tech-transfer offices can help investigators overcome this hurdle. One such program is the NCI TTC Invention and Development Program, which provides additional funding to NCI intramural faculty to perform proof-of-concept studies that can help de-risk their technology and facilitate partnerships with industry. The National Institute of Neurological Disorders and Stroke and the National Center for Advancing Technology Sciences have similar programs to move early-stage technologies forward.
Though there remains some lack of awareness of technology-development opportunities at the NIH, many investigators are working with their tech-transfer offices to move their discoveries to the market. In 2018 alone, the NIH entered into 82 CRADAs, executed 298 licenses to NIH inventions, and obtained 94 newly issued U.S. patents. But NIH “would like there to be more relationships” between NIH labs and industry, said Rohrbaugh.
Investigators are encouraged to sit down with their tech-transfer coordinators and describe their ideas, said Salgaller. “That’s the first step.”
To find your institute’s technology-development coordinator and start the conversation, visit the Office of Technology Transfer website at https://www.ott.nih.gov/tdcs. In addition, on June 12, all are welcome to attend the third annual Technology Showcase, hosted by NCI TTC, at the Frederick National Laboratory for Cancer Research. For more information and to register, go to https://techtransfer.cancer.gov/2019-Technology-Showcase.
Note: Another effort to promote the development of promising drugs or technology is the recent establishment of the NCI-CCR Office of Translational Resources, which acts as an advisor to PIs on how to develop their findings, helps them find commercial partners, and provides resources to make the next steps.
This page was last updated on Monday, April 4, 2022
