Steven A. Rosenberg, M.D., Ph.D.
An Immunotherapy Pioneer Tells All
Steven Rosenberg is widely considered the father of cancer immunotherapy. His 40-year scientific journey has led to an explosion of immunotherapy treatments for numerous cancer types both at the NIH and across the globe.
His journey began when he witnessed one of the rarest events in medicine—the spontaneous regression of a tumor. Early in his career, he had encountered a young man whose cancer had disappeared. Rosenberg, who’s now the chief of surgery in the National Cancer Institute (NCI), believed that the answer had to lie in the patient’s own immune system. (See video.)

CREDIT: RHODA BAER
Dr. Steven Rosenberg, chief of Surgery in the National Cancer Institute, pioneered the development of the first effective immunotherapies for patients with advanced cancer.
Rosenberg started his pioneering work on immunotherapy in the late 1970s when almost nothing was known about T lymphocyte function in cancer and there was no convincing evidence that any immune reaction existed in patients against their cancer. Shortly after the description of a T-cell growth factor now called interleukin 2 (IL-2), Rosenberg began studies of the ability of IL-2 to generate cells with anti-cancer activity in the laboratory and in tumor-bearing mice. In a series of clinical trials based on these findings, he injected IL-2 or cells grown in IL-2 into patients with advanced cancer who had progressed through all available therapies. In the first 66 patients with metastatic cancer in whom the treatment was tried, there was no sign that it worked and all of these patients died of progressive cancer. Then in 1984, Rosenberg treated his 67th patient with a new regimen of high-dose IL-2 and this patient experienced a complete cancer regression that is ongoing 33 years later. These studies of IL-2 administration led to the first FDA approval of a cancer immunotherapy in patients with renal cancer in 1992 and in metastatic melanoma in 1998.
“There is a possibility that an experimental therapy is never going to work,” said Rosenberg. “But when it works once then you know it’s possible.”
To understand the mechanism underlying the ability of IL-2 to cause the regression of cancer in patients with metastatic melanoma, Rosenberg identified immune cells found in cancer tumors called tumor infiltrating lymphocytes (TILs) that had cancer-fighting properties. In a series of clinical trials, Rosenberg and his group were the first to show that the administration of these TILs extracted from a tumor and grown in the laboratory to large numbers could mediate tumor regression in patients with advanced melanoma.
The second big break for immunotherapy was Rosenberg’s discovery that T cells could be genetically modified to detect and destroy cancer in patients. One type of these gene-engineered cells are named chimeric antigen receptor (CAR) T cells, and they were featured on the Discovery Channel’s three-part documentary series First in Human in August 2017. For CAR T-cell therapy to work, the T cells need to be harvested from the patient and outfitted with receptors that recognize surface proteins on the cancer cells. Hematological cancers such as lymphoma and melanoma reside in B cells, which express the cluster of differentiation 19 (CD19) protein. When T cells are modified to be able to identify and destroy B cells expressing CD19, the cancer is destroyed, but so is the normal B-cell population. Luckily, people can live without B cells as long as they get regular infusions of immunoglobulins.
The final piece of the puzzle includes wiping out the patient’s original immune system with chemotherapy, which creates an environment in which the modified T cells can be multiplied within the patient’s body and get to work. In 2009, Rosenberg’s group was the first to successfully treat a lymphoma patient with CAR T-cell therapy, and that patient remains cancer free to this day.
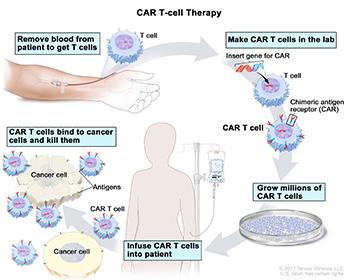
CREDIT: © 2017 TERESE WINSLOW LLC, U.S. GOVT. HAS CERTAIN RIGHTS
CAR T-Cell Therapy (clockwise from top left): Blood is removed from a vein to get T cells; a special receptor called a chimeric antigen receptor (CAR) is made in the laboratory; the gene for CAR is inserted into the T cells; millions of CAR T cells are grown; CAR T cells are infused into the patient; and the CAR T cells bind to cancer cells and kill them.
Rosenberg’s clinic can only treat about six patients per month. To help more people, he needed to collaborate with an industry that could mass produce the therapy. So, in 2012, NCI signed a Cooperative Research and Development Agreement (CRADA) with Kite Pharma, a pharmaceutical company founded by Rosenberg’s former NCI trainee Arie Belldegrun and recently purchased by Gilead Sciences. The company can produce enough CAR T cells to treat 4,000 to 5,000 hospital patients per year.
Kite replicated Rosenberg’s findings in 101 patients from 22 institutions, with more than 40 percent of patients with refractory lymphoma experiencing complete responses—meaning all signs of cancer disappeared.
“The institutions would obtain the patient’s blood cells, send them to Kite Pharma, [which] would introduce the anti-CD19 CAR gene, and then send it back to the hospital’s pharmacy and [the CAR T cells] would get dispensed to patients,” said Rosenberg. It sounds simple, but “behind every discovery that reaches a person there’s a long history of success and failures.” After the replication study, Kite submitted an Investigational New Drug (IND) application to the FDA in 2014, and its protocol was approved in October 2017 thus enabling the treatment to be applied throughout the United States. Rosenberg’s and NCI’s goal is to wipe out cancer. Getting the treatment to the maximum number of patients is the best way to make that happen.
As for the future of cancer research, Rosenberg said, “We are working on approaches now that are really exciting: cell-transfer immunotherapy for patients with any type of cancer.” Current FDA-approved immunotherapies produced by companies like Kite/Gilead are successful in treating only specific types of hematological cancer such as acute lymphoblastic leukemia and lymphoma because the CAR T cells can recognize molecular targets expressed on a certain type of cell’s surface, such as CD19 on B cells. But in the grand scheme of things, epithelial solid cancers such as esophageal, colon, and ovarian cancers account for about 90 percent of cancer fatalities.
With advances in genetic sequencing, scientists and clinicians can now appreciate how complicated cancer really is. Almost every cancer in every patient has a different genetic blueprint, with unique mutations recognized by the immune system. These differences explain why cancer vaccinations and other therapies to target common mutations in patients have failed: There’s no one-size-fits-all treatment.
What Rosenberg’s group is doing now fits the definition of personalized medicine: Target the T cells to the mutations specific to the cancer in each individual. A patient’s tumor is run through genomic sequencing to detect all the mutations in the cancer and identify the TIL that recognize these mutations. These TILs are expanded in culture and reinfused into the patient. “We can find a way to attack the unique patient’s mutations that caused the cancer,” said Rosenberg. “It’s ironic that the mutations that caused the cancer may turn out to be the cancer’s Achilles’ heel and enable successful treatments.”
On the way to Rosenberg’s sizeable corner of the NIH Clinical Center, there is a long hallway with framed portraits of the fellows he has trained over his long and productive career. Each portrait is signed and contains a sentence or two. This wall serves as a reminder that Rosenberg isn’t curing cancer by himself but is leading a battalion of cancer warriors within and beyond the walls of NIH.
What does it take to lead the NCI’s fight against cancer, or for anybody to make substantial progress in a complicated field? Rosenberg recommends passion, but a more precise label might be obsession.
“You have to be living what you’re doing, thinking of the problem in the shower or when stopped at a red light; immerse yourself in the knowledge of a field,” he said. You also have “to define the problem you’re trying to solve in just one sentence, and pursue it with a laser-like focus.”
Rosenberg presented “Cells as Anti-cancer Drugs Entering Mainstream Oncology” at the 12th Annual Philip S. Chen Jr. Distinguished Lecture on Innovation and Technology Transfer in Masur Auditorium (Building 10) on November 17, 2017. Chen spent his career at NIH pioneering ways to deliver ideas discovered at NIH to the outside world. He is credited with forming the NIH Office of Technology Transfer and the CRADA. The recorded videocast can be viewed at https://videocast.nih.gov/launch.asp?23596.
STEVEN A. ROSENBERG, M.D., PH.D.
Senior Investigator, Head, Tumor Immunology Section, Center for Cancer Research, National Cancer Institute
Born: New York City
Grew Up: Bronx, New York
Research Interests: Pioneered the development of effective immunotherapies and gene therapies for patients with advanced cancers. His current research is aimed at extending cell-transfer immunotherapy to patients with common solid cancers.
Education: Johns Hopkins University, Baltimore (B.A. in biology; M.D.); Harvard University, Cambridge, Mass. (Ph.D. in biophysics)
Training: Residency in surgery, Peter Bent Brigham Hospital, Boston; research fellowship in immunology, Harvard Medical School; clinical associate, Immunology Branch, NCI
Came to NIH: In 1970–1972 for training; returned in 1974 as chief of NCI’s Surgery Branch
Thoughts on working at NIH: The NIH Clinical Center remains the world’s greatest research hospital, marrying clinical and basic research. “We provide the best available care but our mandate is not only to practice the best of today’s medicine but to create the medicine of tomorrow.”
Competition: His whole lab is invited (and usually attends) rounds every Monday at 7:30 a.m. Clinical rounds provide fellows with an opportunity to see the clinical application of their research and to keep their efforts in perspective. There’s nothing like seeing patients who hope you have the cure they’ve been waiting for to motivate researchers to push their work to the next level. It’s also a reminder that the squabbling among rival labs shouldn’t weigh on researchers. “Competition seems silly when there’s so much human suffering; there is no sense of competition except against the disease: cancer.”
Outside interests: Enjoys spending time with his family
Family: Has three daughters: One is an attending physician in Emergency Medicine at Temple University Hospital (Philadelphia); one is a life coach in Los Angeles; and one is a bear biologist in Alaska. His wife recently retired after 50 years as a nurse, much of it working with HIV-infected patients.
Website: https://irp.nih.gov/pi/steven-rosenberg
This page was last updated on Friday, April 8, 2022
