The SIG Beat
NEWS FROM AND ABOUT THE SCIENTIFIC INTEREST GROUPS
NEW SIG: SCIENTIA ET PHILOSOPHIA INTEREST GROUP
The Scientia et Philosophia Interest Group seeks to foster and expand the knowledge and understanding of the NIH research community and staff of the philosophical foundations of the scientific endeavor. In an interdisciplinary, open, and inclusive environment, the group promotes an exchange of knowledge in a diversity of fields and topics including the philosophy, origins, and foundations of science; logic and rationalism; cosmology and cosmogony; biology and biogeny; ethics, meta-ethics, and metaphysics; and history of philosophy (classical to modern).
The group stresses how our current empirical scientific projects in basic and clinical research are inseparably tethered to these philosophical underpinnings and are strengthened when clearly grounded on a strong philosophical foundation. A good working knowledge of the philosophical foundations of science and the limits of rationalism allow for better formulation of scientific experimental design, model construction, and parsimonious extraction of inferred conclusions. This SIG is open to intramural investigators, staff, and trainees as well as to extramural affiliates and academic scientists and clinicians outside the NIH. Activities will include regular discussion meetings, internal and external speakers, and webinars. To learn more, join the SCIENTIA_ET_PHILOSOPHIA LISTSERV or contact the group moderator, Peter Leeds (leedsusa@mail.nih.gov).
LIGHT MICROSCOPY INTEREST GROUP
The Light Microscopy Interest Group (LMIG), which aims to build a bridge between NIH biologists and microscopists, informs the NIH community about cutting-edge research in light fluorescence microscopy and about available resources, both extramural and intramural.
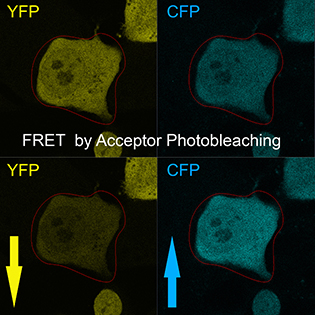
CREDIT: T.S. KARPOVA, NCI
The Light Microscopy Interest Group focuses on innovative microscopy techniques and their application to biomedical research. Shown: A technique called Förster resonance energy transfer (FRET) reveals mammalian cells transformed with the fusion protein cyan fluorescent protein (CFP)–yellow fluorescent protein (YFP) before and after acceptor (CFP) photobleaching.
Modern fluorescence microscopy goes beyond simple observation and localization of single proteins or cellular organelles within cells. It offers the unique advantage of biophysical quantification of intracellular processes in individual live cells, such as diffusion, binding rates, and protein dimerization by fluorescence recovery after photobleaching (FRAP), Förster resonance energy transfer (FRET), single-molecule tracking (SMT), fluorescence correlation spectroscopy (FCS), number and brightness (N&B), and FRET anisotropy imaging (homoFRET). This information may benefit a wide variety of cell-biology research including cancer-related projects.
The light-microscopy field has grown tremendously due to the development of new fluorophores and fast and sensitive imaging systems that are capable of detecting them. Fluorescence markers such as fluorescent proteins and self-labeling fluorescent tags have been developed to observe proteins, lipids, DNA, and RNA in live and fixed cells. Specific fluorescent probes (biosensors) were developed to observe protein interactions and dynamics of enzymatic activity by FRET. Modern light microscopy is enhanced by sensitive, digital cameras, which facilitate the visualization of dim objects, computerized image processing, and quantitative analysis. Specialized techniques such as highly inclined laminated optical sheet (HILO) microscopy were designed to observe individual molecules of proteins. The quality of imaging and resolution may be improved by computational deconvolution and by super-resolution based on structural illumination microscopy (SIM), photoactivation localization microscopy (PALM), or stimulated emission depletion microscopy (STED).
NIH researchers are at the leading edge of this fast-growing field. Do you want to learn more about madSTORM, the super-resolution technique multiplexed antibody size–limited direct stochastic optical reconstruction microscopy that was developed in NCI’s Center for Cancer Research and applied to localization of 25 proteins simultaneously in activated T cells (Chang “Jason” Yi, NCI)? Or how about innovative dyes for single-molecule imaging permitting bright, stable, and reliable labeling of protein fusions to HaloTag, SNAP tag, and CLIP tag (Luke Lavis, Howard Hughes Medical Institute); a new antibody-based drug-delivery method based on “uncaging” of biologically active molecules by near-infrared light (Martin Schnermann, NCI); or genome organization and its cell-to-cell variation revealed by high-throughput fluorescence in situ hybridization (FISH; Elizabeth Finn, NCI)?
If you are interested, come to the LMIG seminars to learn about innovative microscopy techniques and their application to biomedical research with a focus on single-cell imaging and in situ biophysics. The aim is to demonstrate applicability of the state-of-the art microscopy to tissue- and cell-biology problems. Presentations include biological data and in-depth description of appropriate microscopy techniques.
The group moderators are Tatiana Karpova (NCI), Christian Combs (NHLBI), Susan Garfield (NCI), and Ulrike Boehm (NCI). To get the mailings, join the LISTSERV. For more information, contact Chris Combs (combsc@mail.nih.gov) and Tatiana Karpova (karpovat@mail.nih.gov).
LMIG Seminars
- 11:00 a.m.-noon, usually on the third Tuesday of the month (except for November and December)
- Location: Building 37, conference room (4107/4041)
- More information at https://confocal.cancer.gov/LMIG
May 16: Vinay Swaminathan (NIH/NHLBI), “Fluorescence Polarization to Study Mmolecular Anisotropy and Orientation in Focal Adhesions of Migrating Cells.”
June 20: Ronald Germain (NIH/NIAID), “Imaging Immunity–Developing a Spatiotemporal Understanding of Host Defense Using Intravital Dynamic and Multiplex Static Microscopy”
September 19: Steven Vogel (NIAAA), “Investigating the Mechanism of Ultra-fast Energy Transfer in Venus Oligomers Using Time-resolved Anisotropy, Fluorescence Correlation Spectroscopy, and Photon Antibunching“
October 17: Petr Kalab (Johns Hopkins University), “Fluorescence Lifetime Imaging Microscopy (FLIM) for Quantitative Live-cell Measurements with Forster Resonance Energy Transfer (FRET) Probes”
November 14: Kandice Tanner (NCI), “Probing the Physical Pproperties of the Microenvironment in Vivo”
December 12: Ulrike Boehm (NCI), “4Pi‐RESOLFT Nanoscopy: Nanometer Scale 3D Fluorescence Imaging in Whole Living Cells”
More information can be found at the recently renovated website: https://confocal.cancer.gov/LMIG.
This page was last updated on Monday, April 11, 2022
