NIEHS Team Reports How Oxidative Stress Kills Cells
A Key to Understanding the Origins of Some Human Diseases
Humans need energy to function, so it might be hard to imagine how a naturally occurring process that generates power for the body can also harm its cells. But it does, noted Samuel Wilson and members of his NIEHS DNA Repair and Nucleic Acid Enzymology Group.
Based on work done by Wilson’s research fellow Melike Caglayan, the team determined more details about how this particular type of damage leads to DNA strand breaks and, ultimately, cell death. The scientists used biochemical and cell-biology methods, along with X-ray crystallography, to uncover the inner workings of cells. Their recent findings, published in the journal Nature Communications, may help scientists better understand the origins of some human diseases (Nat Commun 8:14045, 2017; doi: 10.1038/ncomms14045).
Breaking DNA: Wilson said that cellular mitochondria, which are specialized energy-producing machines that fuel cells, also produce molecules that harm cellular DNA and proteins. These harmful molecules, known as reactive oxygen species (ROS), can alter the chemical composition of the compounds used to build DNA, as well as DNA itself. If the DNA building blocks are not restored to their original shape, or if DNA is structurally modified due to ROS, the DNA can break, triggering the cell to self-destruct.
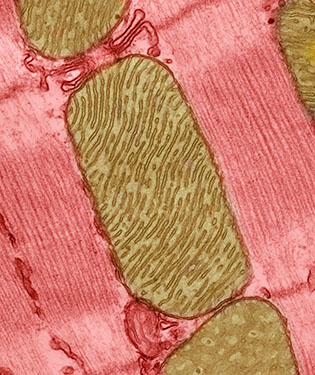
CREDIT: THOMAS DEERINCK, NATIONAL CENTER FOR MICROSCOPY AND IMAGING RESEARCH, UNIVERSITY OF CALIFORNIA, SAN DIEGO
Mitochondria are specialized energy-producing machines that fuel cells, but they also produce reactive oxygen species (ROS) molecules that harm cellular DNA and proteins. Shown: mitochondria (brown) from the heart muscle cell of a rat.
“When ROS-modified compounds are incorporated into a DNA strand, they cause frayed ends that can’t be properly glued together during DNA repair,” Wilson said. “This gap or break in DNA can initiate cell death.”
Double-edged sword: The pressure that ROS places on cells is called oxidative stress, and it happens all the time. The body takes advantage of the killing power of ROS in a series of steps known as innate immunity, which is the natural immunity a person is born with.
For example, Wilson explained that when a bacterium enters the body, a white blood cell activates a special immune cell called a macrophage. The macrophage douses the bacterium with ROS. Just as ROS causes breaks in human cellular and mitochondrial DNA, it will go to work breaking the bacterium’s DNA. That way, the macrophage kills the bacterium before engulfing it.
The scheme is a wonderfully resourceful way to kill living things that could make a person sick, but what happens when the invader is not alive? Take, for example, the particles in cigarette smoke or smog. When these small pieces of matter enter human lung cells, they trigger a similar ROS response by macrophages.
The macrophages use the same mechanism to get rid of invaders. But in this case, the oxidative stress eventually leads to fibrosis, which is the thickening or scarring of tissue seen in chronic lung disease. Wilson mentioned other conditions, such as cataracts, cardiovascular disease, and some neurodegenerative disorders, that are also linked to the effects of oxidative stress.

CREDIT: STEVE MCCAW, NIEHS
Based on work done by Wilson’s research fellow and the paper’s first author, Melike Caglayan, the team determined how oxidative stress damage leads to DNA strand breaks and, ultimately, cell death.

CREDIT: STEVE MCCAW, NIEHS
Advances in structural biology made it possible for Samuel Wilson and his team to demonstrate the subtle way cells can accommodate the oxidative-stress damage inflicted by reactive oxygen species molecules.
Understanding the mysterious mitochondria: Wilson said the research described in the new paper demonstrates a subtle way cells can accommodate the damage ROS inflicts on them. The self-destruction of an individual cell as a result of oxidative stress is a less extreme outcome than the result of exposure to a strong oxidizing agent, such as bleach. In that case, all of the cells in the mix—both the body’s cells and the pathogens—would die.
“We know that the mitochondria probably produce the majority of ROS in a cell,” said NIEHS mitochondrial expert Bill Copeland, who was not involved in this research. “So fully understanding their function may lead to the root of human illness.”
This article is adapted, with permission, from the March 2017 issue of Environmental Factor: https://www.niehs.nih.gov/news/newsletter/2017/3/feature/feature-2-oxidative/index.htm.
This page was last updated on Monday, April 11, 2022
