Celebrating Intramural Science
Report from the 2015 NIH Research Festival
The 2015 Research Festival featured all the usual attractions—scientific talks and workshops, poster sessions (including the institute directors’ and scientific directors’ posters and cooking contest), special exhibits on intramural resources, the Green Labs Fair, the FARE Awards ceremony, the Technical Sales Association exhibit tent show, and more. But this year the festival, which was held on September 16–18 and chaired by the Deputy Director for Intramural Research Michael Gottesman, kicked off the initiatives outlined in the intramural long-term plan. Following are descriptions of the three plenary sessions (in years past, there’s been just one). The concurrent workshops are highlighted in the article at https://irp.nih.gov/catalyst/v23i6/research-festival-selected-symposia-2015.
To view video casts of the plenary sessions:
- Plenary I (September 16) “Creating NIH Technology Incubators,” http://videocast.nih.gov/launch.asp?19154
- Plenary II (September 17): “Responding to Public Health Emergencies,” http://videocast.nih.gov/launch.asp?19159
- Plenary III (September 18): “Chronic Inflammation,” http://videocast.nih.gov/launch.asp?19161
Technology Incubators at the NIH: Bringing Ideas to Light (Plenary I)
Maybe you don’t think of NIH as a hot startup, but there it’s been at the forefront of technology development that intertwines with and supports broader basic- and translational-research efforts. The opening Plenary Session of the 2015 NIH Research Festival brought several innovators—researchers who develop cutting-edge technologies–to the podium, delving into some of the newest frontiers in imaging.
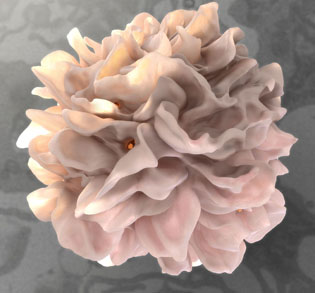
CREDIT: DONALD BLISS, NLM
At the Research Festival’s plenary session on “Creating NIH Technology Incubators,” Sriram Subramaniam described high-resolution electron microscopy and other advanced imaging technologies. Shown: A high-resolution 3D image of a dendritic cell that has captured HIV virions (red dots; center one is most visible) to transfer to a T cell.
The session, said Deputy Director for Intramural Research Michael Gottesman in his opening remarks, “illustrates the critical role that the intramural research program has played over the years, and will play in the future, in developing new technology and innovating.”
The first presentation of the morning began with a clinical problem: How to biopsy for prostate cancer in a way that targets the right place? Although the standard of care is ultrasound-guided biopsy, this method can’t visualize tumors, just the gland itself. Peter Choyke, the director of the National Cancer Institute’s (NCI’s) Molecular Imaging Program, began his presentation with the story of how he, along with many collaborators in the intramural program, devised new ways to pair previously taken magnetic resonance imaging (MRI) information with ultrasound in real time. This technique lets clinicians see where potential tumors are during a biopsy, increasing the chances of hitting the right spot and thus getting a more accurate diagnosis. “We’re on the road at least for improving the diagnostic capabilities for prostate cancer,” said Choyke.
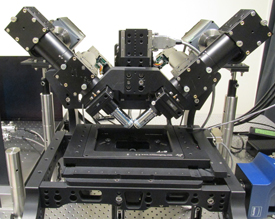
IMAGE BY: MIN GUO, ABHISHEK KUMAR, AND YICONG WU, NIBIB
Hari Shroff has developed this light-sheet microscope to image living organisms very quickly at high resolution as well as in three dimensions (3D) for long durations with multiple colors. The point of all these features is to map, in real time, the growth of individual neurons in the developing brain of the Caenorhabditis elegans worm embryo.
Next up was Hari Shroff (National Institute of Biomedical Imaging and Bioengineering), who has been designing new ways to image living organisms with light microscopy very quickly at high resolution as well as in three dimensions (3D) for long durations with multiple colors, and all at the same time. The point of all these features is to map, in real time, the growth of individual neurons in the developing brain.
Using the Caenorhabditis elegans worm embryo as a model system, Shroff’s light-sheet microscope maps neuronal cells as they grow along the length of the embryo, even keeping track of them after the worm’s muscle cells develop and the embryo starts to twitch and spin. “But all of this technology is useless if it’s siloed in my lab,” he said. Shroff is currently creating a shared advanced-imaging facility where such precommercial custom-built machines can be made available to the rest of the NIH.
Sriram Subramaniam (NCI) rounded out the presentations by discussing technologies for imaging at the smallest end of the scale, such as electron microscopic 3D imaging of cells, viruses, and proteins. The focus, said Subramaniam, is on how the technologies can get you closer to understanding biological mechanisms. Although Subramaniam’s group has both used and developed a variety of electron microscopy-based technologies over the years, the most exciting recent advances have been in cryo-electron microscopy in which the newest generation of cameras has revolutionized the field.
“You can now begin to push the envelope of information that can be collected in single microscopic images,” said Subramaniam. The collected information can lead to structures that reveal the placement of amino acid side chains, ions, and even individual water molecules. “That we can see these types of features is really exciting,” he added, because imaging such features is needed to start to understand chemical mechanism.
But the biggest issue is that there is a gap between people who first try out a technology and those who make it a productive addition to the scientist’s toolkit. “How do we enhance the bandwidth of these technologies at the NIH?” Subramaniam asked.
To answer that question–or at least to delve more deeply into it–the three speakers were joined by Adriaan Bax (National Institute of Diabetes and Digestive and Kidney Disorders), who works with the latest generation of nuclear magnetic resonance technologies; Jennifer Lippincott-Schwartz (National Institute of Child Health and Human Development), who develops super-resolution light-microscopy methods; and Jodi Black (National Heart, Lung, and Blood Institute, NHLBI), deputy director for NHLBI’s Division of Extramural Research Activities, who supports the adoption of new technologies by industry. The difficulty, the panelists agreed, is that although some innovators have been highly successful, the organization of the NIH doesn’t necessarily always support the collaborative, interdisciplinary work required for technology development.
“If we really want to develop something for public-health benefit,” said Black, “you’ve got to be rewarded for moving into something that’s commercially relevant.”
“The recognition of people who are innovators, inventors, toolmakers, at the same or higher level of credit as we give to our scientists who are studying biological processes” is a part of the long-term goal of the intramural program, said Gottesman. “We need to recognize that they are really essential parts of teams that are solving important problems.”
To view a videocast of Plenary Session I “Creating NIH Technology Incubators,” go to http://videocast.nih.gov/launch.asp?19154.
Ebola Epidemic and Beyond: An Overview of NIH’s Health Emergency Response (Plenary II)
When the Ebola epidemic in West Africa became a global health emergency in 2014, NIH stepped in to help develop a clinical-research program in the region. In addition, the NIH Clinical Center’s Special Clinical Studies Unit treated several health-care workers who were exposed to or infected by Ebola virus either in West Africa or in the United States.
But this occasion was “not the only time NIH has played a central role in responding to an outbreak,” NIH Director Francis Collins said in his opening remarks during the Research Festival’s plenary session on “Responding to Public Health Emergencies.”

PHOTO BY: LISA HELFERT
A panel discussion followed each of the plenary sessions. Here, the “Responding to Public Health Emergencies” panel featured (from left) David Lipman (NCBI), Pamela Collins (NIMH), Cliff Lane (NIAID), NIAID Director Anthony Fauci, and NIEHS Director Linda Birnbaum.
NIH routinely responds to emergencies whether in the United States or elsewhere, from the Fukushima nuclear disaster in Japan (2011) to Hurricane Katrina in New Orleans (2005).
“The NIH intramural community as a group has come together to try to deal with things of global importance, the way that they have in the setting of Ebola,” said Cliff Lane, deputy director for Clinical Research at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID took the lead role in helping NIH’s effort to battle Ebola. Lane has taken over a dozen trips to West Africa since the epidemic started; more than 100 staff from the intramural research program (IRP) from 14 different institutes have traveled there as well.
NIH brought together experts from the NIH intramural and extramural communities and paired them with experts in Liberia to establish a clinical-research infrastructure to tackle Ebola. It will have the sustainability to study future public-health challenges as well. NIH has been conducting clinical trials on several Ebola vaccine candidates and experimental therapeutic interventions such as the monoclonal-antibody cocktail ZMapp. Currently studies are active in Liberia, Sierra Leone, and Guinea.
But not all epidemics are as obvious as Ebola. One silent epidemic is mental illness, which “is the leading cause of disability around the world,” said Pamela Collins, associate director for Special Populations at the National Institute of Mental Health (NIMH). Unfortunately, “very little has been done to remedy it,” she said. There is a gap between the number of people in need of mental-health care and the number who receive it, even in the United States. To reduce the gap, which is due in part to shortages in mental-health providers, NIMH has established collaborative hubs in different parts of the world that are testing “task-shifting” approaches in which less-specialized care providers deliver evidence-based mental-health interventions.
David Lipman, scientific director of the National Center for Biotechnology Information (NCBI), discussed NIH’s role in developing a national genomics network for food safety. Although the mortality rate from foodborne illnesses is low, the associated health-care costs exceed $20 billion. NCBI analyzes and manages the publicly accessible data for the collaboration among CDC, FDA, and state public-health laboratories—the first such network to use whole-genome sequencing for identifying outbreaks of foodborne pathogens. In the future, the full genome sequence for both viral and bacterial pathogens can help detect foodborne outbreaks earlier, according to Lipman.
The panel discussion that followed the presentations included remarks by National Institute of Environmental Health Sciences (NIEHS) Director Linda Birnbaum and NIAID Director Anthony Fauci. Birnbaum described NIEHS’s disaster research-response program, which involves a cross-institute effort. NIEHS also responds to public-health emergencies related to environmental pollution, climate change, and electronic waste. In addition, an NIH disaster interest group meets monthly to come up with better strategies to deal with emergencies.
Fauci asked about the IRP’s role in building a sustainable infrastructure in Africa. Lane explained that the NIH goal is to build a program that can eventually be led and maintained by the West African investigators and their institutions.
Deputy Director for Intramural Research Michael Gottesman wanted to know whether the intramural research community could in any way help Pam Collins’ effort to address the mental-health issues. Collins said that she hopes that one day experienced nurses from the intramural community will be available to supervise other nurses in mental-health programs.
NIH’s long-term plan is to “think about ways in which the NIH could more effectively harness the enormous talent” from the NIH intramural community to deal with public-health emergencies, said Gottesman.
The “Responding to Public Health Emergencies” plenary session can be viewed at http://videocast.nih.gov/launch.asp?19159.
Chronic Inflammation (Plenary III)
In the plenary session on “Chronic Inflammation,” intramural researchers discussed the identification and therapeutic targeting of a central immune-signaling pathway, advanced-imaging techniques in multiple sclerosis (MS), and the development of the Center for Human Immunology, Inflammation, and Autoimmunity (CHI). The session culminated in a panel discussion of how the NIH can promote collaborative investigations of chronic inflammation.
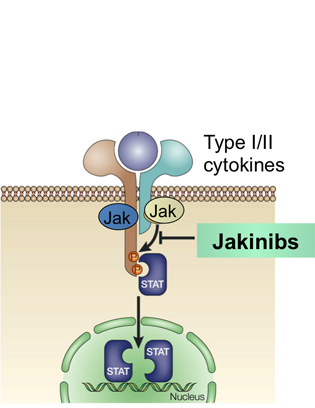
CREDIT: NIH DIVISION OF MEDICAL ARTS
In the “Chronic Inflammation” plenary, John O’Shea described the Jak-STAT pathway as a simple and rapid membrane-to-nucleus mechanism for transmitting extracellular signals. JAK kinase inhibitors are currently approved for therapeutic use, including tofacitinib for rheumatoid arthritis.
John O’Shea (National Institute of Arthritis and Musculoskeletal and Skin Diseases, NIAMS) described the mechanisms of cytokine signaling and associated clinical applications. He discovered the tyrosine kinase Janus kinase 3 (JAK3) and contributed to work showing that JAK3 associates with the common gamma chain, a receptor component used by several cytokines, and that immunodeficiencies arise from mutations in this signaling pathway. Three JAK kinase inhibitors are currently approved for therapeutic use, including the JAK inhibitor tofacitinib for rheumatoid arthritis, which was developed in partnership with Pfizer. Cytokine-mediated JAK–STAT (signal transducer and activator of transcription protein) signaling plays a central role in determining T-cell fate. O’Shea’s current research is focused on how cytokines regulate lineage-specific gene expression and epigenome through which these factors determine T-cell identity.
Daniel Reich (National Institute of Neurological Disorders and Stroke) discussed advanced imaging for MS. MS is characterized by autoimmune T cells that attack myelin, the insulating layer of nerve cells, leading to specific zones of demyelination, called lesions, in the brain. Recent advances allow mapping of the formation and repair of these lesions in patients. These advances are able to discriminate differences in lesion-formation patterns, which may offer clues about the early stages of lesion repair. One pattern in particular is characterized by chronically activated, pro-inflammatory macrophages around the edge of the lesion, which may arrest the wound-healing process, resulting in more extensive tissue damage. In addition to classifying lesions, these new imaging modalities provide a means to identify therapeutic interventions that favor the healing and remyelination of lesions.
In the final talk, CHI director Neal Young described how the center uses advanced-technology platforms, as well as data integration and computational modeling, to work with diverse collaborators across the NIH intramural program to study the human immune system in health and disease. Angélique Biancotto discussed the technical platforms available at CHI. Integration of these platforms with clinical data may expedite the identification of novel correlates and biomarkers of disease. John Tsang discussed systems immunology and the importance of assessing the immune system for precision medicine. One of the first major CHI studies used a vaccine as a model for assessing immune responsiveness after challenge. This study identified signature subpopulations of immune cells present before, challenge that are predictive of vaccine responsiveness. Ultimately, these approaches may reveal signatures common to several diseases and immune reactions.
In the panel discussion, led by Michael Gottesman, O’Shea, Reich, and Young, Rachel Caspi (National Eye Institute),NIAMS Director Stephen Katz, and Thomas Wynn (NIAID) discussed the enhancement of resources for chronic-inflammation research including improved access to central-core facilities; the creation or expansion of facilities such as a germ-free mouse facility; and the advantages and disadvantages of grouping researchers of like interests together. Several leadership initiatives were put forth including the need for resources to support interested fellows; possible mechanisms of joint appointment for investigators wishing to work in more than one institute; and the potential benefits of earlier collaborations with consortium companies. In addition, the Inflammatory Disease Interest Group will host a minisymposium on inflammation, fibrosis, and tissue regeneration on May 2, 2016, in Lipsett Amphitheater (Building 10); and a new bimonthly inflammation seminar series will commence on November 10, 2015.
NIH’s collaborative approach to research aims to elucidate global principles of chronic inflammation that underlie many disease processes. It is hoped that the discovery of these principles will translate into more precise therapies for patients.
To view the videocast of the Research Festival’s “Plenary III: Chronic Inflammation,” go to http://videocast.nih.gov/launch.asp?19161.
To join the Inflammatory Disease Interest Group, go to https://list.nih.gov/cgi-bin/wa.exe?SUBED1=INFLAM-DIS-L&A=1.
This page was last updated on Monday, April 25, 2022
