Research Briefs
NHLBI: ENTEROVIRUSES DEPEND ON CHOLESTEROL FOR REPLICATION
Researchers at NHLBI discovered that the Enterovirus genus of viruses—which includes human pathogens such as polioviruses, Coxsackie viruses, and rhinoviruses (which cause the common cold)—depend on cholesterol for replication. These viruses actively rewire the endocytic membrane trafficking pathways of host cells to maximize cholesterol absorption and delivery to their replication platforms. The findings have significant implications for the treatment of individuals with enteroviral infections; treatment and management of individuals with high cholesterol concentrations, who may be more susceptible to enteroviral infections; and treatment and management of individuals with type 1 diabetes, in whom high cholesterol concentrations and enteroviral infections are frequently observed together. By targeting the viral and host factors that are required for cholesterol absorption and delivery to the replication platforms (as identified in this study), scientists may be able to develop panviral therapeutics that can block the replication of many different enteroviruses in humans. (NHLBI authors: M. Santiana, W.-L. Du, Y.-H. Chen, N. Altan-Bonnet; Cell Host Microbe 14:281–293, 2013)
NICHD: NEW DRUG COULD SLOW PROGRESS OF FATAL CHILDHOOD DISORDER
Batten disease is a fatal, inherited disorder of the nervous system that typically begins in childhood. NICHD researchers have identified a potential new drug that could help in the treatment of a form of the disease. When tested in mice, the drug slowed the loss of coordination seen in the disorder and extended the animals’ life span. The drug is derived from hydroxylamine, a molecule chemically similar to ammonia. Hydroxylamine is toxic, but a slight change in the molecule’s chemical structure results in a nontoxic molecule, called NtBuHA, short for N-(tert-Butyl)-hydroxylamine. The researchers hope NtBuHA will be useful for treating a particular subtype of the disease, infantile Batten disease. Children with this form of the disease have a genetic deficiency of the enzyme palmitoyl-protein thioesterase-1, which ordinarily breaks down ceroid, a waxy substance. The researchers are also evaluating two other drugs—cysteamine bitartrate (Cystagon) and acetylcysteine (Mucomyst)—for the treatment of the disease. (NICHD authors: C. Sarkar, G. Chandra, S. Peng, Z. Zhang, A. Liu, and A.B. Mukherjee; Nature Neurosci 16:1608–1617, 2013)
NIAID, VRC: NIH SCIENTISTS DEVELOP CANDIDATE VACCINE AGAINST RESPIRATORY SYNCYTIAL VIRUS
An experimental vaccine to protect against respiratory syncytial virus (RSV), a leading cause of illness and hospitalization among very young children, elicited high concentrations of RSV-specific antibodies when tested in animals, according to NIH researchers. Early-stage human clinical trials of the candidate vaccine are planned. The scientists built on their previous findings about the structure of a critical viral protein to design the vaccine. Earlier this year, the VRC team obtained atomic-level details of an RSV protein—called the fusion (F) glycoprotein—bound to a broadly neutralizing human RSV antibody. The protein-antibody complex gave scientists their first look at the F glycoprotein as it appears before it fuses with a human cell. In its prefusion shape, F glycoprotein contains a region vulnerable to attack by broadly neutralizing antibodies (antibodies able to block infection from the common strains of RSV).
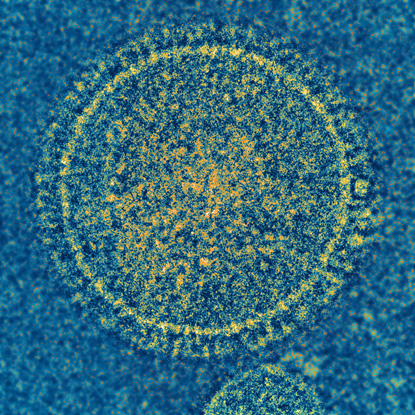
NIAID
The respiratory syncytial virus (RSV) is responsible for a common childhood illness. NIAID and VRC scientists have developed a candidate vaccine that show promise in animals and plan to conduct early-stage clinical trials in the future.
Once RSV fuses with a cell, this vulnerable area, named antigenic site zero by the researchers, is no longer present on the rearranged F protein. In natural RSV infection, the immune system produces antibodies against both the prefusion and postfusion forms of F glycoprotein. But the antibodies to antigenic site zero, which is only present on the prefusion form, have much stronger neutralizing activity. Therefore, a vaccine against RSV would have greater chance of success by eliciting antibodies directed at F glycoprotein in its prefusion configuration.
The researchers described how they used this structural information to design and engineer F glycoprotein variants that retained antigenic site zero even when no antibody was bound to it. The goal was to create stable variants that could serve as the foundation for a vaccine capable of eliciting a potent antibody response. The researchers designed more than 100 variants; of these, three were shown by X-ray crystallography to retain the desired structure. The engineered variants were then used as vaccines in a series of experiments in mice and rhesus macaques. It turned out that the more stable the protein, the higher the concentration of neutralizing antibodies elicited by vaccination.
The scientists are continuing to refine the engineered F glycoproteins and hope to launch early-stage human clinical trials of a candidate RSV vaccine as soon as clinical-grade material can be manufactured. (NIH researchers: J.S. McLellan, P.D. Kwon, B.S. Graham, and others; Science 342:592–598, 2013; and NIH researchers: J.S. McLellan, P.D. Kwon, B.S. Graham, and others; Science 340:1113–1117, 2013)
NIEHS: TANNING GENE LINKED TO INCREASED RISK OF TESTICULAR CANCER
A gene important in skin tanning has been linked to higher risk for testicular cancer in white men, according to a study led by scientists from NIEHS and the University of Oxford in England. Nearly 80 percent of white men carry a variant form of this gene, which increased risk of testicular cancer as much as threefold in the study. The research is the result of an integrated analysis of “big data” (a collection of databases too large and complicated to be easily manipulated in traditional ways) supported by laboratory research. The team suspected that variations in a gene pathway controlled by the tumor suppressor gene p53 could have both positive and negative effects on human health. The G allele of the KIT ligand oncogene (KITLG) is associated with a greater risk of testicular cancer and is more frequent in whites than Africans or those of African descent.
The p53 stimulates skin tanning when ultraviolet light activates it in the skin. It then must bind a specific sequence of DNA located in KITLG, which stimulates melanocyte production, causing the skin to darken. To conduct the analysis, NIEHS researchers led a data mining expedition to sieve through many different datasets. The team selected possible leads from the intersection of more than 20,000 p53 binding sites in the human genome, 10 million inherited genetic variations genotyped in the 1000 Genomes Project, and 62,000 genetic variations associated with human cancers identified in genome-wide association studies. These datasets were gathered through joint efforts of thousands of researchers from around the world. (NIEHS authors: J.X. Wang, M.R. Campbell, D. Su, and D.A. Bell; Cell 155:410–422, 2013)
NIDDK, CIT: FIRST DETAILED ANALYSIS OF MOLECULAR STRUCTURE OF AMYLOID PLAQUES
Researchers have long assumed Alzheimer disease (AD) to be caused by abnormal protein deposits called amyloid plaques in the brain, but until recently have not known much about molecular structures of the amyloid-beta (A-beta) fibrils within these plaques. NIDDK and CIT investigators, together with colleagues from the University of Chicago, have now developed methods for determining the molecular structures of A-beta fibrils in brain tissue of AD patients, based on solid-state nuclear magnetic resonance spectroscopy. The investigators examined fibrils grown from brain tissue of two AD patients with different clinical histories and found that each patient had developed a single fibril structure, but that fibril structures in the two patients were different from each other. Moreover, fibrils that developed in brain tissue were different from fibrils grown in the test tube.
The work represents the first detailed analysis of the molecular structures of the deposits that develop in AD patients’ brains. It is estimated that about five million Americans have AD, the most common cause of dementia in older people. At present, the illness can be definitively diagnosed only after death, by linking clinical measures with an examination of brain tissue and pathology in an autopsy. Information gathered from examining molecular structures of A-beta fibrils may provide a way to accurately diagnose mild cognitive impairment in still-living AD patients, allowing for early intervention and potential inhibition of disease progression. The findings pave the way for new patient-specific strategies to improve diagnosis and treatment of this common and debilitating disease. (NIH authors: J.-X. Lu, W. Qiang, W.-M. Yau, C.D. Schwieters, and R. Tycko; Cell 154:1257–1268, 2013)
This page was last updated on Thursday, April 28, 2022
