Helping the Heart Stand Up to Sepsis
NIH Researcher Explores Why Some Survive Infection-Induced Organ Damage
NIH research is shedding light on what happens to the heart when the immune system over-reacts to an uncontrolled infection, a condition known as sepsis.
“When you have eliminated the impossible, whatever remains, however improbable, must be the truth.” That line from Arthur Conan Doyle’s Sherlock Holmes can be applied to mysteries of all sorts, including the ones scientists toil away in their labs to solve. When it comes to solving the many mysteries of sepsis — a life-threatening immune over-reaction to an uncontrolled infection — the process of elimination is leading us closer to answers, thanks to researchers at the NIH Clinical Center.
Sepsis — also known as septic shock in its most severe form — occurs when the body’s immune system kicks into overdrive to fight a severe infection. Unfortunately, rather than just attacking the harmful invaders, the immune system releases chemicals that, when present in excess, cause intense, tissue-damaging inflammation and impair organ function. In recognition of Sepsis Survivors Week, we spoke with IRP Senior Investigator Charles Natanson, M.D., about two of the great mysteries of sepsis: how does sepsis cause organ failure in the first place, and why do some people survive it while so many others die?
“Sepsis is not a disease, it’s a syndrome, and when something is a syndrome, as opposed to a disease, it indicates that we don’t really understand what is going on,” says Dr. Natanson, who has been studying sepsis’ effects on the heart since arriving at NIH in 1981. “What happens is you get septic shock, multi-organ failure, and other harmful metabolic changes in the body that seem to be separate effects from those occuring at the site of infection itself.”
When organ damage from sepsis is severe enough, the organ may no longer be able to function properly. If the patient survives, that damage may heal over time, but as many as 60 percent of survivors end up with lasting physical and cognitive effects. Dr. Natanson studies how sepsis can cause a condition called cardiomyopathy in surviving patients, in which the heart’s muscular walls weaken and lose the ability to pump blood effectively.

Dr. Charles Natanson
According to Dr. Natanson’s research, unlike other organs that sustain damage from sepsis, the heart appears to stick to a strict schedule for healing, typically returning to healthier levels of function after two weeks in those who survive.
The mystery of what actually causes the organ damage and what makes the difference between survival and death has puzzled researchers like Dr. Natanson for years. While unchecked inflammation itself may be the cause, researchers have also suggested that other chemical and physical effects separate from the inflammatory immune response could push organ injury over the precipice and reduce chances of survival. However, Dr. Natanson’s lab has been studying these other changes and, he believes, eliminated many of them as suspects.
“We’ve looked at blood flow to the heart and it’s normal or even increased after sepsis,” he explains. “We’ve also looked at stress hormones, and the injury to the heart occurred whether they were present in excess or not. And we found inflammatory triggers can reproduce the injury and anti-inflammatory agents can reduce it. So, we think the data suggest that inflammation is the likely cause.”
While his initial studies back in the 1980s were limited by the technology available at the time, Dr. Natanson and his colleagues are now able to study cardiomyopathy’s physical damage and repair at an atomic level in an animal model. By making repeated scans over two weeks on a magnetic resonance imaging (MRI) machine borrowed from a colleague, he and his team were able to observe the repair process and measure changes in the heart.
Dr. Natanson’s research has benefited immensely from magnetic resonance imaging (MRI) technology like the scanner above due to its ability to produce high-quality images of what is going on inside the body during and after sepsis.
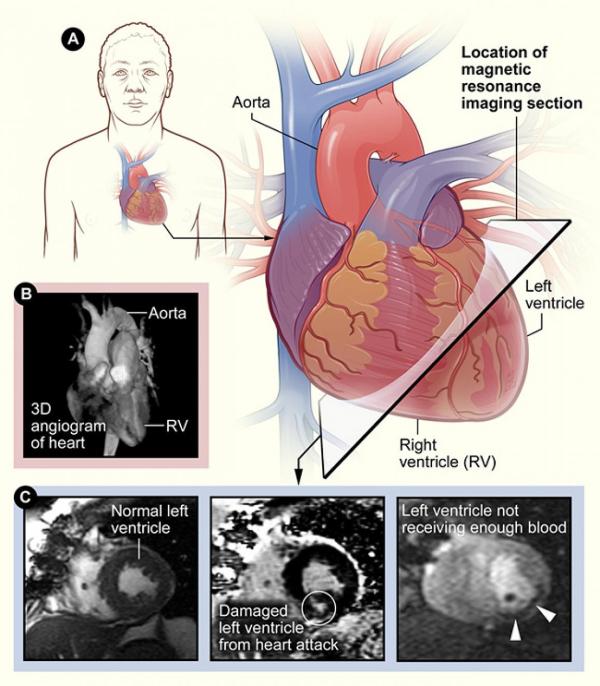
Some example images of the human heart produced via MRI.
The high-quality internal images of animal models of sepsis that MRI produces have helped his team to identify three physical changes that may affect survival: how much weight the heart loses; the severity of injury to the heart’s smallest blood vessels and muscle cells; and the ability of the heart chamber wall to expand and contract once it begins to heal. The larger the tissue loss and damage, and the less flexible the heart is, the lower the odds of survival.
One of the more interesting findings to come from Dr. Natanson’s studies — and one that could only be made using MRI — was that the heart’s flexibility after sepsis is profoundly influenced by the ratio of water to actual cellular tissue in its walls. Intriguingly, unlike a sinking ship, it’s actually a good thing for the heart’s walls to become relatively more water-logged, since removing damaged tissue but retaining the water in a wall makes it more ‘compliant,’ meaning it is better able to stretch as it fills with blood and contract to pump the blood elsewhere. When this process fails to make the walls more compliant, sepsis is lethal.
“In relative terms, the water takes up the same amount of wall space that was originally there and only the dry mass molecules are lost,” Dr. Natanson explains. Normally, he says, we think of tissue taking on water and swelling, but in this case it appears that the damaged protein molecules in the heart are removed while water content is not lost, so the heart’s total water content remains the same. Previous studies, which didn’t use MRI, couldn’t show that the heart was losing more weight from shedding damaged tissue than it was from losing water.
“What we found was unusual, but we’re using MRI, which measures the water mass on an atomic level to make these observations,” he adds. “We’re measuring the amount of water and non-water and it’s extremely accurate.”
As more evidence falls into place, Dr. Natanson believes that identifying inflammation as the likely culprit behind organ tissue damage and establishing the timeline of healing could eventually allow doctors to treat sepsis much more effectively by severing the connection between the uncontrolled infection and the organ damage that typically results.
“I'm pretty excited about our discoveries,” Dr. Natanson says. “I don’t think we’ve proven that inflammation is the main cause, but there's no alternative explanation that fits our findings, and I do believe we've really made a better, more biologically plausible argument to explain the process of organ injury in sepsis, which has never been fully understood before.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, February 19, 2025
