A Promising New Tool to Combat Antibiotic-Resistant Bacteria
IRP Research Demonstrates the Perks of Overlooked Immune System Molecules
More and more bacteria like this E. coli are evolving ways to survive conventional antibiotic treatments, but new IRP research points to a possible solution to that problem.
Countless Hollywood romances rely on the trope that, every so often, it turns out the thing you’ve been searching for has been right in front of you all along. Thanks to new IRP research, a molecule long-studied for its role in directing immune cells’ movement around the body is being seen in a new light as a potentially important player in medicine’s life-or-death struggle against antibiotic-resistant infections.1
Despite scientists’ best efforts, bacteria that shrug off conventional antibiotics continue to infect more than 2.8 million people in the U.S. each year, killing 35,000 of them, according to the U.S. Centers for Disease Control and Prevention (CDC). Consequently, researchers are searching everywhere they can think to look for new ways to kill bacteria, including within our own bodies. For instance, scientists have known since the beginning of the new millennium that short proteins, or peptides, called chemokines that our immune system produces can directly kill infectious microbes like bacteria. However, their main role in the body is to direct immune cells to the site of infections so that those cells can attack the harmful invaders, and most research on chemokines has therefore focused on how they do that.
“That function of chemokines kind of obscured their function as antimicrobials, and for many scientists, this second, antimicrobial activity was almost considered like an accident,” explains Sergio Pontejo, Ph.D., the new study’s first author and a staff scientist in the lab of IRP senior investigator Philip Murphy, M.D.
“The list of peptides that are used as antibiotics is, I think, zero,” adds Dr. Murphy, the study’s senior author. “They’re hard to deliver, they get degraded by the body so they don’t last long in the bloodstream, and they’re harder to manufacture and make money from, so there hasn’t been a big push from the pharmaceutical industry to develop peptides as antibiotics. And if you’re interested in studying the biological activity of chemokines, there’s so much redundancy with other antimicrobial proteins that it’s very difficult to show what they do inside the body, and it’s hard to get a grant to fund studies of their activity in petri dishes.”

Dr. Sergio Pontejo (left) and Dr. Philip Murphy (right)
Fortunately, pursuing research in the IRP comes with the freedom to perform those kinds of narrowly tailored experiments in order to reveal the intricacies of biological molecules like chemokines. For example, several years ago, Dr. Murphy and Dr. Pontejo showed that chemokines can bind to components of cell membranes called phospholipids, which Dr. Pontejo describes as “the essential bricks” of the cell membranes that act like the skin of both human and bacterial cells.2 The discovery made Dr. Pontejo wonder if chemokines’ ability to directly kill bacteria might depend on their interaction with phospholipids in the bacterial cell membrane, so in the new study, he and his collaborators investigated that possibility. They found that only chemokines that can bind to negatively charged phospholipid molecules are able to kill bacteria in test tubes. What’s more, two such chemokines were better at killing E. Coli bacteria than a peptide found in our bodies called beta-defensin 3 that specializes in killing infectious microbes.
Several additional experiments reinforced the importance of negatively charged phospholipids for chemokines’ antimicrobial abilities. For instance, the scientists showed that an antimicrobial chemokine binds mostly to the parts of the bacterial cell membrane that contain the highest concentration of two negatively charged phospholipids, cardiolipin and phosphatidylglycerol. Moreover, when the scientists added antimicrobial chemokines to test tubes with bacteria and liposomes, lab-created particles made of different combinations of phospholipids, they found that only liposomes made of cardiolipin or phosphatidylglycerol reduced the chemokines’ ability to kill the bacteria. This suggests that the chemokines were binding to the liposomes rather than the bacteria, but only when the liposomes were made up of negatively charged phospholipids.
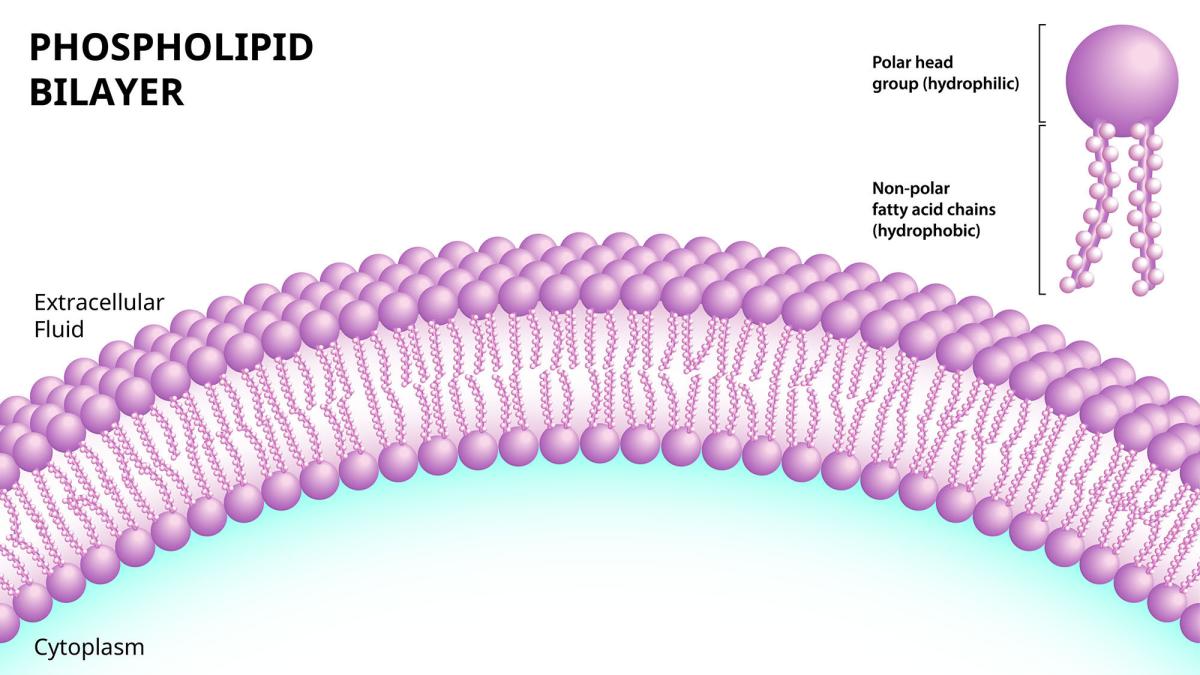
Cells are surrounded by a barrier of phospholipids that separate the fluid inside them, known as cytoplasm, from the outside world. Those phospholipids consist of a head that is ‘hydrophilic,’ meaning it attracts water, attached to a ‘hydrophobic’ chain that repels water. By slicing apart negatively charged phospholipids like cardiolipin, certain chemokines create lethal leaks in bacterial cell membranes.
Finally, the researchers demonstrated that antimicrobial chemokines are very good at killing a type of bacteria that has both cardiolipin and phosphatidylglycerol in its cell membrane, but noticeably less effective at destroying a version of that bacterium that cannot make cardiolipin, so it only has phosphatidylglycerol in its cell membrane. That difference occurred because the antimicrobial chemokines were better able to break apart the cell membrane of the first type of bacteria than the second type.
“It’s simple to understand: the chemokines that kill bacteria bind to the membrane of the bacteria, which contains cardiolipin, and they break the membrane,” Dr. Pontejo says. “Bacteria cannot survive without their membranes.”
Perhaps even more importantly, treating bacteria with an antimicrobial chemokine called CCL20 did not cause the microbes to become resistant to the chemokine. The researchers tested this by exposing groups of bacteria to doses of CCL20 or one of two conventional antibiotics that were high enough to kill many but not all of the bacteria, then transferring the survivors in each group to a new test tube to repopulate, after which they were exposed again to the same chemokine or antibiotic. Over multiple generations, higher and higher doses of the antibiotics were needed to kill significant numbers of the bacteria previously exposed to the antibiotics, but the dose of CCL20 needed remained the same.

Whenever a group of bacteria is exposed to an antibiotic, a few may survive. With their antibiotic-susceptible brethren no longer around to compete for limited resources, that small contingent of antibiotic-resistant bacteria can multiply rapidly, producing large numbers of similarly hardy descendants.
“Chemokines attack the negatively charged membranes of bacteria, while antibiotics attack proteins or channels or enzymes that bacteria need to survive,” Dr. Pontejo says. “The membranes are made up of lipids, and it’s more difficult for bacteria to modify those lipids to become resistant to chemokines, whereas if you’re targeting an enzyme, a bacteria can more easily develop mutations that make that enzyme immune to antibiotics.”
Taken together, the study’s findings suggest scientists may have made an error in largely ignoring chemokines’ antimicrobial properties for the past two decades. Now that we know not only that bacteria don’t become resistant to chemokines after being exposed to them, but also that chemokines kill bacteria by ripping apart the negatively charged phospholipids in their cell membranes, researchers like Dr. Pontejo and Dr. Murphy can zero in on the parts of antimicrobial chemokines that bind to those phospholipids and use what they learn to create lab-designed peptide molecules that single-mindedly seek out and destroy bacteria.
“The problem now is that chemokines can also bind to immune cell receptors to attract those cells and they can bind to sugar molecules on the surface of our cells,” Dr. Pontejo explains. “If you want them to bind to cardiolipin in order to kill bacteria, those other functions might interfere with their ability to do that. If we can find a pattern in chemokines’ structure that is common amongst all chemokines that can bind to cardiolipin, we can cut down the peptides we’re making to the one essential part that they need to bind to and kill the bacteria.”
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
References:
[1] Pontejo SM, Martinez S, Zhao A, Barnes K, de Anda J, Alimohamadi H, Lee EY, Dishman AF, Volkman BF, Wong GCL, Garboczi DN, Ballesteros A, Murphy PM. Chemokines kill bacteria without triggering antimicrobial resistance by binding anionic phospholipids. Sci Adv. 2025 Jun 6;11(23):eads2675. doi: 10.1126/sciadv.ads2675.
[2] Pontejo SM, Murphy PM. Chemokines act as phosphatidylserine-bound "find-me" signals in apoptotic cell clearance. PLoS Biol. 2021 May 26;19(5):e3001259. doi: 10.1371/journal.pbio.3001259.
Related Blog Posts
This page was last updated on Tuesday, August 5, 2025
