A New Tool in the Battle Against Depression
Annual Lecture Details Revolutionary Treatment’s Bench-to-Bedside Journey

Nearly two decades of IRP research resulted in a drug that quickly curbs symptoms of depression even in the most difficult-to-treat patients.
As hard as IRP scientists work in the lab, they work equally hard to make sure their findings have a real-world impact on patients’ lives. The pathway from the lab to the clinic, though, is rarely straightforward — something IRP senior investigator Carlos Zarate Jr, M.D., knows first-hand from his game-changing innovations in treating depression.
Dr. Zarate closed this year’s NIH Research Festival on September 22 by describing that odyssey in the 16th annual Philip S. Chen, Jr., Ph.D. Distinguished Lecture on Innovation and Technology Transfer. Named in honor of the former IRP investigator who established NIH’s Office of Technology Transfer in 1986, the annual event celebrates important IRP innovations that have moved beyond the boundaries of NIH.
The new treatment for depression that Dr. Zarate’s IRP team developed — a chemical called ketamine — was all the more important because research on the topic had long been stagnant. Depression affects nearly 300 million people around the world, but most of the commonly used antidepressants brought to market over the past few decades have been what Dr. Zarate calls “copycat drugs” because they all work by manipulating the same brain systems. While those medications undoubtedly offer relief to many people with depression, repeatedly aiming drugs at the same target is a less-than-ideal strategy for treating an illness that varies widely between patients.
"There are hundreds of ways to meet criteria for major depressive disorder, and that has been one of the biggest problems in drug discovery,” Dr. Zarate said during his lecture. “We don’t talk about one depression, but hundreds of depressions.”
Dr. Zarate at the NIH Clinical Center
Unsurprisingly, then, many patients have depression that is ‘treatment-resistant,’ remaining mired in hopelessness no matter which of those medications they take. What’s more, conventional treatments for depression can take weeks or months to begin improving symptoms.
“You’re asking your patients to hang in there, that things will get better, but they’re having disruption to their personal and social life and are at risk for suicide,” Dr. Zarate explained.

Despite the plethora of antidepressants brought to market in the second half of the 20th century, many patients with depression still don’t find relief from their symptoms.
Dr. Zarate came to NIH in 2000 to lead a team that sought to develop new treatments for depression. Rather than retread the same ground as the copycat drugs, he and his colleagues decided to go in an entirely new direction. They would spend the next two decades exploring ketamine’s potential as a rapid-acting therapy for patients with treatment-resistant depression.
Ketamine’s chemical structure is similar to that of PCP, which was originally used to put people under before surgery but was banned in the 1960s due to its mind-altering side effects. Ketamine was developed in the 1950s with the idea that it could be similarly used as anesthesia but with less potent and long-lasting side effects. The U.S. Food and Drug Administration (FDA) approved ketamine in 1970 for that purpose, and it subsequently found widespread use in a variety of medical and veterinary settings, as well as in combat zones during the Vietnam War.
Over the next four decades, scientists determined that ketamine blocks the ‘NMDA’ receptors found on some neurons. They also found that ketamine and other molecules that block NMDA receptors improve symptoms in animal models of depression, even when administered at ‘sub-anesthetic’ doses much lower than those used to put surgical patients under.
Based on those preclinical findings, as well as the results of a small, previously published pilot study testing ketamine in only seven patients with depression, Dr. Zarate launched a clinical trial at the NIH Clinical Center in 2004 to assess its effects in a larger group of individuals with treatment-resistant depression. In contrast to the slowly emerging effects of conventional antidepressants, a single intravenous infusion of ketamine at a sub-anesthetic dose improved the symptoms of more than two-thirds of those patients within hours.
“That was really rewarding to see that in our earlier patients and still to this day,” Dr Zarate said. “It never goes away, that feeling.”
In this video from 2013, Dr. Zarate discusses his efforts to develop new, rapid-acting treatments for depression.
Multiple follow-up clinical trials conducted by Dr. Zarate’s team and other groups confirmed that those results were no fluke, and the findings began drawing attention from both the media and pharmaceutical companies. Building on this work, Dr. Zarate’s team also found that ketamine had antidepressant effects in people with bipolar depression. Perhaps most impressive was the finding that a single, low dose of ketamine markedly reduced suicidal thoughts, an effect that appeared within just 40 minutes and lasted as long as a week.
“Which is huge, in a sense,” Dr. Zarate said. “That gives you time in emergency room settings, in patient hospitalizations, to transfer patients back to their home or get treatment from their treatment providers in the community.”
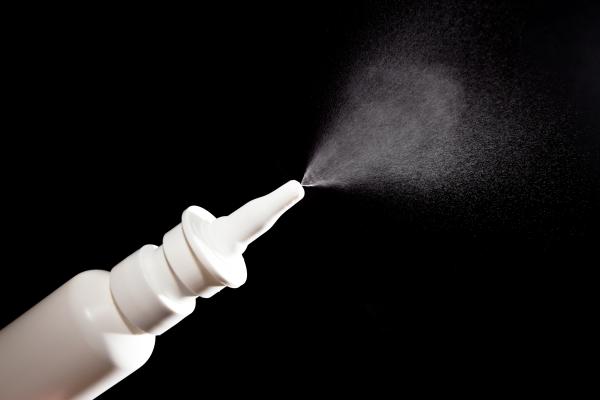
Dr. Zarate’s research on ketamine led to the FDA approval of esketamine, a form of ketamine that can be delivered via nasal spray. This approach reduces the number of medical personnel needed to administer the treatment and lowers its cost.
However, despite those compelling findings, ketamine has a significant downside: it must be administered intravenously and requires nurses and anesthesiologists to monitor the patient in a clinical setting, which is “not practical for psychiatrists or nurse practitioners in the community,” Dr. Zarate said. Consequently, his lab collaborated with researchers at other institutions and with Janssen Research and Development — a division of pharmaceutical company Johnson and Johnson — to create a version of ketamine that could be administered with a nasal spray.
Janssen decided to focus on a form of ketamine known as S-ketamine, or esketamine, and the resulting treatment — marketed as Spravato — was approved by the FDA for adults with treatment-resistant depression in 2019 and for adults with strong suicidal thoughts in 2020. The European Medical Agency also approved Spravato for the same uses in 2019 and 2021, respectively. As a nasal spray, Spravato can be administered in a variety of settings by just one person trained in its proper use.
Even after his success in helping to bring an entirely new type of antidepressant to the market, Dr. Zarate is not resting on his laurels. He is now investigating the antidepressant properties of hydroxynorketamine (HNK), one of more than 20 molecules the body produces as it processes ketamine. Research by Dr. Zarate’s IRP team and its collaborators over the past seven years has shown that HNK also has rapid antidepressant effects in mice but does not appear to cause the same mild mind-altering side effects as ketamine. Based on these exciting results, Dr. Zarate’s team is planning to launch a clinical trial in the coming months to test whether HNK can improve symptoms in patients with treatment-resistant depression.
“We can only do so much in preclinical studies,” Dr. Zarate said. “We have to test it in patients to see if that’s the case.”
It took nearly two decades to turn ketamine into a groundbreaking new treatment for depression, and HNK likely faces its own long road ahead. Nevertheless, such long-term efforts are well worth it. Not only was the development of ketamine as a highly effective and rapid-acting antidepressant hailed as one of the most significant developments in the field of psychiatry over the past few decades, it also re-energized the field of drug development for psychiatric illnesses. Ketamine’s journey from bench to bedside stands as an inspiring example of how collaborations between NIH and the pharmaceutical industry can lead to life-changing therapeutics for people suffering from devastating diseases.
Subscribe to our weekly newsletter to stay up-to-date on the latest breakthroughs in the NIH Intramural Research Program.
Related Blog Posts
This page was last updated on Wednesday, October 4, 2023
