The Reservoir Within: How Nitrate Secures Our Nitric Oxide Supply
The Nitrate–Nitrite–NO Cycle
In the annals of metabolic biochemistry, some molecules grab all the glory. Nitric oxide (NO), a reactive gas with outsized influence on blood flow, neurotransmission, and immunity, earned “Molecule of the Year” honors in 1992 from Science magazine and was the subject of a 1998 Nobel Prize.
But what of nitrate, NO₃⁻, its more stable cousin, long relegated to the role of inert byproduct and sometime dietary contaminant? Turns out that in mammals, nitrate may pool to serve as a key reservoir for NO production, buffering NO availability for cardiovascular functions and preventing oxidative damage through a cyclic metabolic pathway.
This theory is according to a review article published in April in the journal Nutrients (PMID: 40362853) by NIDDK scientists Barbora Piknova, Ji Won Park, and Alan Schechter of the NIDDK Molecular Medicine Branch. They propose that the nitrate–nitrite–NO cycle is a self-sustaining system that maintains tissue perfusion and blood flow under varying physiological conditions.
Additionally, they propose that organs such as skeletal muscle and skin are the locations of important nitrate reservoirs, ensuring NO bioavailability during external or internal stress, which broadens the understanding of how NO homeostasis is regulated beyond the more classically studied enzymatic synthesis.
In short, nitrate—once dismissed as a mere metabolic byproduct of NO breakdown—emerges as a vital ally in sustaining life’s most essential functions through its role in this dynamic, reversible cycle.
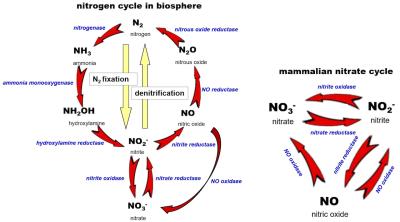
CREDIT: Piknova et al (PMID: 40362853)
A view of the overall pathways involving species in the biosphere is shown in panel a, with the cyclic nitrate-nitrite-NO pathway shown in panel b.
NO as a Positive
The discovery of NO as a vital signaling molecule is itself an interesting irony. NO is a gas regarded historically as a toxic pollutant. Yet through the 1970s and 1980s, scientists began to understand how NO, despite being a short-lived free radical, is crucial for human physiology. Among those behind these revelations were Robert Furchgott, Louis Ignarro, and Ferid Murad (the latter two trained at the NIH), who discovered that NO can be produced enzymatically by a family of NO synthase enzymes from L-arginine.
They demonstrated that NO activates soluble guanylate cyclase in target cells, leading to increased cyclic guanosine monophosphate concentrations and resulting in vasodilation. This discovery fundamentally changed the understanding of cardiovascular physiology. And for this find they shared the 1998 Nobel Prize in Physiology or Medicine.
Meanwhile, in the 1980s and 1990s, Schechter, who leads the NIDDK Molecular Biology and Genetics Section, was studying normal and sickle hemoglobin at a molecular level, including understanding the effects of hemoglobin interactions with various gases, knowledge that later helped inform how hemoglobin interacts with NO.
In particular, through the decade of the 2000s, Schechter and colleagues demonstrated that hemoglobin and red blood cells not only transport oxygen but also play an active role in regulating NO availability in the blood and tissues, thus modulating vascular tone and contributing to the broader understanding of NO biology.
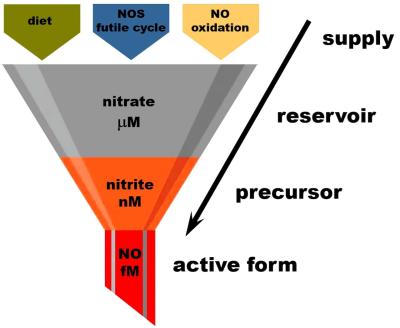
CREDIT: Piknova et al (PMID: 40362853)
Nitrate as an “NO reservoir” molecule.
Getting to NO
Nitrate is one of the primary sources of NO in the body, mostly obtained through consumption of vegetables such as beets and leafy greens. After ingestion, nitrate is absorbed into the bloodstream and concentrated in certain tissues. In the acidic environment of the stomach, through bacterial reduction in the oral cavity or by certain mammalian enzymes, nitrate is converted into nitrite (NO2-), which can then be further reduced to NO in tissues, especially under hypoxic or acidic conditions.
Nitrate seems like a terrible thing to just flush away, yet most of it does indeed wind up in urine and is excreted. How then does the body maintain an adequate supply of nitrate and other NO precursors for when short-lived NO is needed, such as during times of exertion? Piknova, a staff scientist in Schechter’s lab, has pondered this question for some time. In reviewing work by Lundberg et al. (PMID: 19915529) and others on the nitrate–nitrite–NO pathway, she began to wonder about the importance of her finding of large nitrate stores in certain tissues.
Drawing on multiple lines of evidence—such as biochemical measurements, tracer studies, and tissue analyses across various species, including humans—Piknova and her team reference multiple experimental studies that use advanced techniques such as chemiluminescence detection and isotope tracing to measure nitrate and nitrite concentrations in several mammalian tissues and fluids. Specifically, they cite measurements showing high baseline nitrate concentrations in certain tissues, such as skeletal muscle, which can increase significantly following dietary nitrate intake, raising the possibility that the stored nitrate in these tissues can be mobilized and shared among organs when needed.
Framing these tissues explicitly as reservoirs with a central role in NO metabolism and systemic regulation, an example of the classical concept of homeostasis, is a relatively new and significant development in this field, Schechter said.
And this expanded view opens new avenues for nutritional and therapeutic strategies aimed at supporting NO availability, especially in aging, cardiovascular disease, and metabolic disorders where NO pathways are impaired, he added.
“This reservoir concept reflects how the body maintains an overall constant internal environment, a concept that dates back to Claude Bernard’s idea of the milieu intérieur, formulated in the 1840s,” Schechter said. “This idea later evolved into the concepts of homeostasis and cybernetic control, which were central to physiology in the first half of the 20th century but have been less emphasized in the past 60 years with the focus on reductionist studies in specific types of cells and organs.”
Curiously, and perhaps not coincidentally, plants rely on a largely reverse cycle of nitrite-to-nitrate conversion, as seen for example in aquaponics, in which the ammonia in fish urine is converted by bacteria first to nitrite and then to nitrate, which is absorbed by the plants…which the fish then eat. In the reciprocal cycling of nitrogen, life’s many biological forms become co-regulators of their shared environment, illustrating Gaia theory’s premise that Earth’s organisms and systems evolve together to sustain conditions favorable for life.
But will this get you to eat more beets? No or NO?
This page was last updated on Wednesday, July 9, 2025
