Research Briefs
Read about scientific advances and discoveries by NIH intramural scientists: augmented T cells treat solid tumors; a new reference panel captures genomic diversity; brain network uniquely activated through intravenous drug use; heart PET scans may predict Parkinson’s disease and dementia; overdose mortality increased in pregnant and postpartum women.
NCI: CYTOKINE ARMORED T-CELLS EFFECTIVE ON SOLID TUMORS
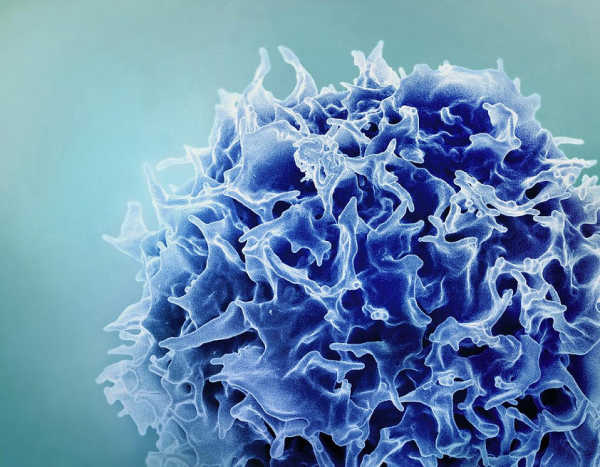
CREDIT: NIAID
Colorized scanning electron micrograph of a T lymphocyte (also known as a T cell)
Chimeric antigen receptor (CAR) T-cell therapy has been effective in treating blood cancer but hasn’t worked as well on solid tumors. In a new frontier of immunotherapy, NCI scientists showed how augmented T cells might successfully treat cervical cancers and pediatric neuroblastoma.
CAR T-cell therapy is a form of cellular immunotherapy that involves engineering T cells in the laboratory so they can specifically target and kill tumors. Researchers orchestrated a more effective immunotherapy by modifying two types of T cells (CAR T cells and T-cell receptor-engineered T cells) to carry cytokines—interleukins 15 and 21—on their surfaces. Cytokines can boost T-cell function but can also cause severe side effects in patients. This new approach delivered the boosted T cells in a more targeted way.
In mouse models of cervical cancer, cancer tumors shrank in up to 80% of mice treated with T cells carrying both interleukins, versus 20% in those treated with T cells carrying only one. Mice in the former group also survived longer and had no serious side effects.
The researchers then conducted the experiment in several clinically relevant and unrelated disease models, including pediatric neuroblastoma, and found similar results. They believe their approach could address the need for better T-cell therapies for patients with solid tumors and aim to eventually study it in human clinical trials. (NIH authors: R. Nguyen, C.M. Mousset, B.Y. Jin, R. Okada, X. Zhang, J.M. Reyes-GonzalezGonzález, L. Zhang, S. Abbas, M. Sun, C. Hsieh, M. Ho, J.F. Shern, and J.L. Gulley, Clin Cancer Res 2023; DOI:10.1158/1078-0432.CCR-23-1872)
[BY SEPPIDEH SAMI, CC]
NHGRI: FAILING TO ACCOUNT FOR MIXED GENETIC LINEAGES COULD LEAD TO INACCURACIES
Not fully accounting for genomic variance in a population may lead to inaccurate study results, according to new research led by NHGRI scientists. When broadly sampling people of European ancestry, the researchers found two false associations involving the lactase gene (which gives people the ability to digest lactose): one between lactase and height, and the other between lactase and cholesterol concentrations.
Using data published in genetic-association studies, the NHGRI team created a new reference panel that captured genomic diversity not previously seen in people of European ancestry. By using the new reference panel, they revealed the inaccurate associations and in contrast, found a genuine true-positive association between the lactase gene and body mass index.
Information about how genomic variants are related to different traits helps researchers estimate polygenic risk scores and may give clues about a person’s ability to respond safely to drug treatments. The new findings highlight the importance of accounting for mixed ancestral backgrounds in genomic studies. According to the authors, it’s likely that there are other false associations in the literature and that some true associations are yet to be found. (NIH authors: M.H. Gouveia, A.R. Bentley, A.A. Adeyemo, C.N. Rotimi, and D. Shriner, Nat Commun 14:6802, 2023; DOI:10.1038/s41467-023-42491-0)
[BY CODY R.K. CONRAD, NIAID]
NIAAA, NIDA, CC: BRAIN NETWORK IS UNIQUELY ACTIVATED THROUGH INTRAVENOUS DRUG USE
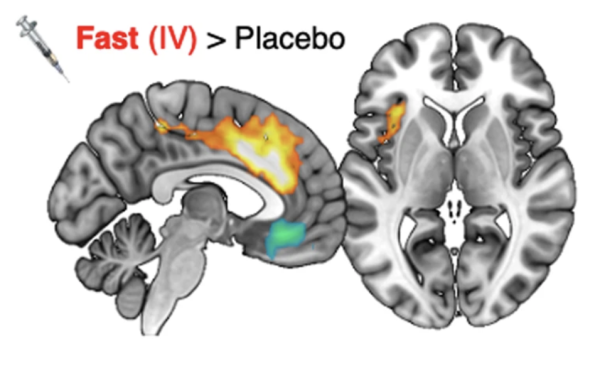
CREDIT: NIAAA, NIDA
NIAAA, NIDA, CC: Whole-brain analysis showing the salience network (orange and yellow) where brain activity was significantly associated with fast dopamine increases after intravenous administration of methylphenidate—an effect not observed with oral administration of methylphenidate.
A team of NIAAA and NIDA researchers at the NIH CC found that intravenous drug administration activates brain regions within the salience network, a neural system responsible for gauging the euphoric effects of a drug. When the same drug was administered orally, the network remained inactive, representing a new discovery in addiction neuroscience.
Using simultaneous positron-emission tomography (PET) and functional magnetic-resonance imaging (fMRI), investigators recorded the brain activity, dopamine signaling, and self-reported feelings of euphoria from 20 participants across three clinical sessions in which intravenous and oral administration of methylphenidate (Ritalin) or a placebo were administered.
The researchers found that two salience-network brain regions were activated in association with the fast dopamine increases to intravenous methylphenidate: the dorsal anterior cingulate cortex and the insula. Participants also reported euphoric effects after intravenous use, which were described as more intense when those regions were more active. These effects were not observed in PET or fMRI imaging with the oral administration of methylphenidate, even when the same peak concentration of dopamine was reached.
Routes of administration that get drugs to the brain fast, such as intravenous injection, maximize the rewarding effects, which makes drugs more reinforcing and in time can lead to more severe addiction. According to the authors, if the salience network can be inhibited during quick drug administration, then it may block the drug’s euphoric effects, giving more insight into the course of addiction and presenting an intriguing new treatment target. (NIH authors: P. Manza, D. Tomasi, E. Shokri-Kojori, R. Zhang, D. Kroll, D. Feldman, K. McPherson, C. Biesecker, E. Dennis, A. Johnson, W. Wang, M. Yonga, G. Wang, and N.D. Volkow, Nat Commun 14:6408, 2023; DOI:10.1038/s41467-023-41972-6)
[BY MEAGAN MARKS, NIAAA]
NINDS, NHGRI: HEART AUTONOMIC PET SCANS PREDICT PARKINSON’S DISEASE AND DEMENTIA
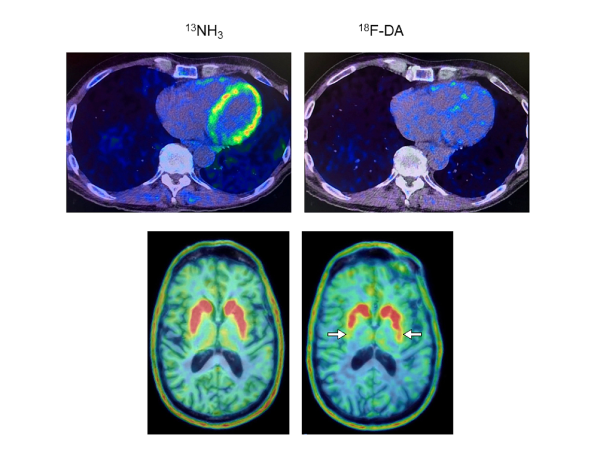
CREDIT: GOLDSTEIN LAB, NINDS
Heart and brain PET scans from a study participant who developed Parkinson’s disease support a “body-first” progression. The top pair shows low 18F-dopamine-derived radioactivity in the heart (right) and a normal 13N-ammonia PET scan (left), which preceded a loss of dopamine-producing neurons and symptom onset.
A team led by NINDS researchers discovered that PET scans of the autonomic nerves supplying the heart may identify people who are at risk of developing central Lewy body diseases (LBDs) such as Parkinson’s disease (PD) and dementia with Lewy bodies.
In this prospective, long-term follow-up study, the authors analyzed data from 34 individuals with PD risk factors over the course of 7.5 years or until PD was diagnosed. Researchers conducted serial 18F-dopamine PET scans of the heart to analyze concentrations of the neurotransmitter norepinephrine. Norepinephrine is derived from dopamine, which is deficient in the brains of individuals with PD. The authors had discovered previously that norepinephrine in the heart is severely decreased in people with LBDs.
Eight of nine participants with low cardiac 18F-dopamine-derived radioactivity at the time of their first scan later developed a central LBD. In contrast, only one of eleven participants with typical initial concentrations of radioactivity later developed disease during the same period of follow-up. All nine participants who developed a central LBD had low radioactivity before or at the time of diagnosis. Among the 25 participants who remained disease-free, only one had persistently low radioactivity.
“The loss of norepinephrine in the heart can precede and predict the loss of dopamine in the brain in Lewy body diseases,” senior investigator and study co-author David S. Goldstein said in a press release. In contrast, individuals with the same PD risk factors but with normal radioactivity are much less likely to develop such diseases.
According to the authors, these results highlight the importance of finding biomarkers that could help early detection and initiate early intervention of brain diseases before symptoms occur. (NIH authors: D.S. Goldstein, C. Holmes, P. Sullivan, G. Lopez, J. Gelsomino, S. Moore, R. Isonaka, and T. Wu, J Clin Invest DOI:10.1172/JCI172460)
[BY ANNELIESE NORRIS, NCI]
NIDA: OVERDOSE MORTALITY INCREASED IN PREGNANT AND POSTPARTUM WOMEN

Credit: Georgetown University
Emily Einstein
In a cross-sectional study of national mortality data, NIDA researchers found that, from early 2018 to late 2021, mortality from drug overdose among pregnant and postpartum people aged 35 to 44 more than tripled, from 4.9 deaths to 15.8 deaths per 100,000 mothers.
The investigators also found that individuals who died from overdose were more likely to be younger, be non-college graduates, and be unmarried compared with people who died of obstetric causes. They were also more likely to have died at home or other non-healthcare setting, consistent with the phenomenon that overdose deaths often occur in drug-consumption locations.
According to the authors, these findings indicate the need for strengthening community outreach and maternal medicine support and point to barriers in pregnant individuals seeking treatment, such as socioeconomic resources, stigma, and fear of loss of child custody. “These results reflect the persistent national overdose crisis and demonstrate that pregnancy is an urgent time for interventions that can reduce the risk of overdose,” said NIDA Science Policy Branch Chief and study co-author Emily Einstein in a press release. (NIH authors: B. Han, W.M. Compton, E.B. Einstein, E. Elder, and N.D. Volkow, JAMA Psychiatry DOI:10.1001/jamapsychiatry.2023.4523)
[BY NAOMI GREENBERG, NHLBI]
This page was last updated on Saturday, November 23, 2024
