COVID-19 Timeline at NIH (March-April 2022)
COVID-19 Research and Activities at NIH
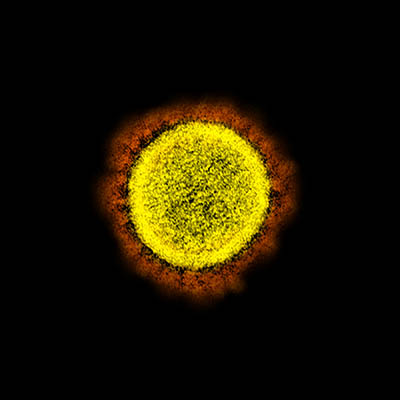
CREDIT: NIAID
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
March 1: NIH implements a new process for staff to voluntarily report COVID-19 vaccinations received in the community.
March 3: The CDC releases updated guidance on when COVID-19 mitigation measures are to be used based on COVID-19 community levels. Both mask wearing and screening and testing are recommended in workplace and community settings when community levels are high. Mitigation measures for high-risk individuals are recommended during medium community levels, and no mitigation measures are recommended during low community levels. HHS will require employees in federal workspaces to comply with the new guidance beginning Sunday, March 6, 2022.
March 4: NIH Acting Director Lawrence Tabak emails staff to report U.S cases of COVID-19 are down 37.7% from the previous week and that the percentage of fully vaccinated Americans is now 76.4%. He reviews the CDC mask wearing and screening and testing guidance updated on March 3 and notes that the guidance for healthcare settings such as NIH has not changed. NIH staff working onsite should continue to wear masks until the NIH Coronavirus Response and Recovery Team assesses the impact of the new guidance on NIH. He announces updated density requirements stating that all NIH facilities across the country may begin operating at 1 person per 35 square feet of space.
March 7: NIH Deputy Director for Management Alfred Johnson announces that the authorization to use excused absence for hours in which employees are unable to work to care for dependents will expire on March 26, 2022. He distributes a new HHS office-space planning policy.
March 9: NIAID begins a clinical trial designed to help understand rare but potentially serious systemic allergic reactions to COVID-19 mRNA vaccines. “This study will help us determine if individuals who experienced moderate systemic allergic reactions can safely receive a second dose of a COVID-19 mRNA vaccine,” said NIAID Director Anthony Fauci.
March 10: An NIH-funded study finds that schools with mandatory masking during the delta variant surge had approximately 72% fewer cases of in-school transmission of SARS-CoV-2 compared with schools with optional or partial masking policies. The study included more than 1.1 million students and over 157,000 staff attending in-person school across nine states. (Pre-publication release: Pediatrics 2022; DOI:10.1542/peds.2022-056687)
March 11: NIH Acting Director Lawrence Tabak emails staff to announce that NIH will implement the March 3 CDC guidance for masking on March 14 and screening testing for unvaccinated persons on March 26, based on COVID-19 community levels at all NIH locations. The safety guidance for health care settings with the potential for patient contact has not changed, where masks will continue to be required. To help staff prepare to return to on-site work, NIH adds a preparation section to the Return to the Physical Workplace intranet page with helpful resources, toolkits, and checklists.
March 18: NIH Acting Director Lawrence Tabak emails staff announcing that the 10th Virtual Town Hall will be held on April 5 with NIH leadership to address the state of the pandemic and answer employee questions about returning to the physical workplace. He mentions this week’s Federal Safer Workforce update for safety protocols for visitors to federal facilities based on COVID-19 community levels: When community level is medium or high in a county where a federal facility is located, visitors to that facility should be asked to provide information about their vaccination status or provide proof of a recent negative COVID-19 test. These requirements do not apply to patients or patient visitors, and NIH is considering how this change affects visitors to NIH. Tabak reports that masking requirements for on-site meetings will follow the CDC guidance based on community levels issued on March 3, and he continues to encourage staff to voluntarily report their vaccination status to the Division of Occupational Health and Safety.
March 21: The NIH Clinical Center (CC) updates its visitation policy to welcome visitors provided they are able to follow hospital requirements, which include screening for COVID-19 symptoms prior to entering the CC, properly wearing the CC-issued medical-grade mask during visits, and maintaining a six-foot distance from others. Masks are required for both adults and children.
March 27: The authorization to use excused absence for hours in which employees are unable to work to care for dependents expires. NIH employees who are unable to complete their normal work hours as a result of dependent care responsibilities, after exercising supervisor-approved flexibilities, are required to use their own approved annual leave, sick leave, or leave without pay, as appropriate.
March 28: All administrative buildings on the NIH Bethesda campus return to their prepandemic building access hours of operation, and approval to come on site is no longer required. Administrative buildings remain locked on nights and weekends and staff will need their PIV card to gain access outside of normal weekday business hours.
March 29: The CDC recommends additional mRNA COVID-19 boosters for individuals who are moderately or severely immunocompromised or over 50 years of age and received an initial booster dose at least 4 months ago. Separately, those who received a primary vaccine and booster dose of Johnson & Johnson’s vaccine at least 4 months ago are now eligible to receive a second booster dose using an mRNA COVID-19 vaccine.
March 31: NIAID sponsors a clinical trial to evaluate whether a second COVID-19 booster shot–including multiple-variant vaccines—can broaden immune responses in adults who already have received a primary vaccination series and a first booster shot.
March 31: NIAID Director Anthony Fauci and his colleagues publish a new perspective that discusses how achieving classical herd immunity against SARS-CoV-2 is unlikely. However, widespread use of currently available public health interventions to prevent and control COVID-19 will enable resumption of most activities of daily life with minimal disruption. (J Infect Dis, jiac109, 2022; DOI:10.1093)
March 31: The SARS-CoV-2 Assessment of Viral Evolution program is described in a paper published in Nature. The effort was a scientific consortium established by NIAID with faculty from the Mount Sinai School of Medicine (New York) and was designed to provide a real-time risk assessment of SARS-CoV-2 variants on immune protection. (Nature 2022; DOI:10.1038/s41586-022-04690-5)
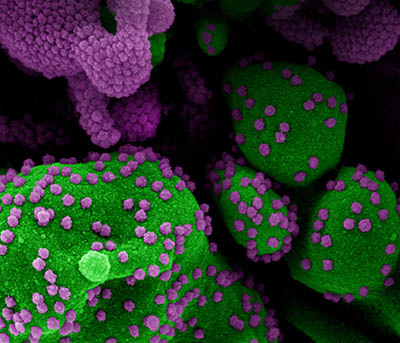
CREDIT: NIAID
Colorized scanning electron micrograph of an apoptotic cell (green) heavily infected with SARS-CoV-2 virus particles (purple), isolated from a patient sample. Image from the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
April 1: In his email to all staff, NIH Acting Director Lawrence Tabak reviews the March 29 CDC recommendation for additional mRNA COVID-19 boosters for certain individuals. He highlights a new website launched by the Biden administration called COVID.gov that serves as a portal for information on vaccines, tests, treatments, masks, and the latest on COVID-19. Tabak mentions that as more staff return to campus, NIH is making efforts to resume operations of cafeterias, gyms, and other amenities. He reminds all employees to respect one another’s decisions to wear or not to wear a mask when mask wearing is optional based on COVID-19 community levels, as assessed by the CDC at all NIH locations on a weekly basis.
April 4: The symptomatic COVID-19 testing car line moves to a tent located east of the B1 cafeteria at Building 10 and will operate Monday–Thursday. Previously, the car line was at two locations. The first location was at the vehicle inspection tent outside the Natcher Conference Center (Building 45) before moving to the Gateway Vehicle Inspection Station (Building 66A).
April 4: The Washington Post reports how research into long COVID is accelerating a revolution in the medical research process by allowing patients, who have typically been only subjects in the research process, to become partners in it. The NIH received $1.15 billion from Congress to launch the four-year RECOVER initiative to understand long COVID and other post-viral conditions such as chronic fatigue by using patient experiences to frame research questions. According to NINDS Director Walter Koroshetz, the NIH initiative is starting clinical trials.
April 5: NIH Acting Director Lawrence Tabak hosts the 10th Virtual Town Hall with NIH leadership to update staff and answer 22 frequently asked questions on the state of the pandemic, workplace flexibilities, and safety guidance as employees return to the workplace. Over 10,000 staff participated in the live event.
April 5: The White House COVID-19 Response Team and HHS public health officials hold a press briefing. CDC Director Rochelle Walensky shares the latest on the state of the pandemic, NIAID Director Anthony Fauci discusses the effectiveness of additional booster shots, and HHS Secretary Xavier Becerra provides an update on work to address the long-term impacts of COVID.
April 6: NIH hosts the first in-person Wednesday Afternoon Lecture Series since March 4, 2020, in the Lipsett Amphitheater. The speaker is Anna Huttenlocher, Professor of Pediatrics and Medical Microbiology and Immunology at the University of Wisconsin at Madison (Madison, Wisconsin). She delivers a talk titled “Imaging Inflammation Resolution and Wound Repair” to a small, invited audience.
April 10: After two years maximum of telework all NIH staff are able return to work under the HHS Return to the Physical Workplace plan. NIH will continue many of the flexibilities that are outlined in the newly issued HHS Workplace Flexibility Policy.
April 11: The Office of Research Services Division of Mail Management Services restarts full mail delivery to all NIH buildings both on and off campus.
April 13: The Transportation Security Administration extends its mask mandate, which was set to expire on April 18, for two more weeks as the CDC monitors the increase in COVID-19 cases. Masks will continue to be required for travel on airplanes, in airports, on buses, and trains, including Metro, VRE, and MARC, through May 3. On April 18, a federal judge in Florida overturns the CDC mask mandate; the administration plans to file an appeal.
April 15: In an email to all staff NIH Acting Director Lawrence Tabak welcomes many employees back to the physical workplace this week. He reports that although the national seven-day average of new COVID-19 cases has increased by 4.9%, hospitalizations and deaths continue to decline. The community transmission levels as reported by the CDC remain low at NIH facilities and Tabak asks staff to be prepared to implement additional workplace mitigation efforts should the community levels change to medium or high.
April 19: The NIH Fitness Centers in Building 53 and Rockledge II reopen for service.
April 25: The NIH Library reopens its doors for the first time after closing on-site services on March 18, 2020. Library Reading Room hours will be Monday through Friday from 8:30 a.m. to 4:00 p.m.
April 27: NIH holds a virtual meeting and listening session on the U.S. government’s Oversight Framework for Research Involving Enhanced Potential Pandemic Pathogens (ePPP). The purpose of this meeting is to get input from a wide range of stakeholders on the scope of the framework, strategies for minimizing potential biosafety and biosecurity risks, considerations for supporting international ePPP research, and how to balance security with public transparency. Members of the public are given the opportunity to register to speak at the meeting.
April 29: Acting NIH Director Lawrence Tabak’s all-staff email reports that yesterday, Moderna asked the FDA to authorize its coronavirus vaccine (which NIH helped develop) for children under 6 for emergency use, making it the first manufacturer to do so.
April 29: After weeks of most NIH locations remaining at low risk in the CDC COVID-19 community levels, Framingham, Massachusetts, rose to medium, raising the specter of other facilities moving to a higher risk level and a return of stricter safety regulations.
This page was last updated on Tuesday, May 17, 2022
