Research Briefs
NCCIH, NIDDK, NICHD: SPECIALIZED NEURONS PLAY A UNIQUE ROLE IN PAIN
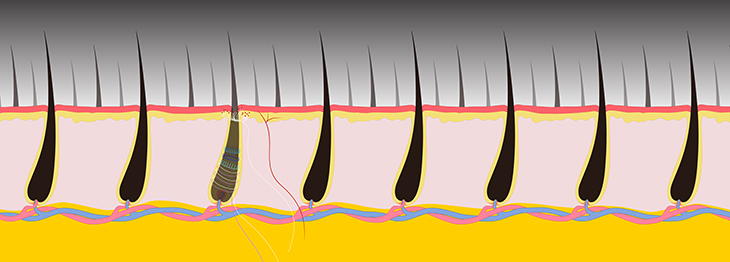
CREDIT: JEREMY SWAN AND NICOLE SWAN, NICHD
Researchers in an NCCIH-led study have identified a class of sensory neurons that can be activated by stimuli as precise as the pulling of a single hair. Understanding basic mechanisms underlying these different types of responses will be an important step toward the rational design of new approaches to pain therapy.
Researchers in an NCCIH-led study have identified a class of sensory neurons that can be activated by stimuli as precise as the pulling of a single hair. Understanding basic mechanisms underlying these different types of responses will be an important step toward the rational design of new approaches to pain therapy. In this study, the researchers used a novel strategy that combined functional imaging, recordings of electrical activity in the brain, and genetics to see how neurons respond to various stimuli. The scientists focused on a class of sensory neurons that express a gene called CALCA because these neurons have a long history in pain research.
The scientists applied various stimuli to the hairy skin of mice cheeks, including gentle mechanical stimuli (air puff, stroking, and brushing), “high-threshold” mechanical stimuli (hair pulling and skin pinching), and temperature stimulation. They found that the target neurons belong to two broad categories, both of which were insensitive to gentle stimulation. The first was a well-known type of pain fiber—a polymodal nociceptor—that responds to a host of high-intensity stimuli such as heat and pinching. The second was a unique and previously unknown type of neuron that responded robustly to hair pulling. They called this previously undescribed class of high-threshold mechanoreceptors (HTMRs) “circ-HTMRs,” due to the unusual nerve terminals these neurons made in skin. The researchers observed that the endings of the fibers made lasso-like structures around the base of each hair follicle.
In additional experiments, the researchers found that using optogenetics to directly activate circ-HTMRs was sufficient to drive protective behaviors such as avoiding a chamber paired with blue-light stimulation. These findings add insight into how the somatosensory system encodes pain. To see videos describing the research, go to https://www.nih.gov/news-events/news-releases/nih-study-uncovers-specialized-mouse-neurons-play-unique-role-pain and http://www.cell.com/neuron/video. (NIH authors: N. Ghitani, A. Barik, M. Szczot, J.H. Thompson, C. Li, C.E. Le Pichon, M.J. Krashes, and A.T. Chesler, Neuron 95:944–954.e4, 2017; DOI:http://dx.doi.org/10.1016/j.neuron.2017.07.024)
NIAID: NIAID RESEARCHERS PIONEER ROBUST NEW 3-D TISSUE-IMAGING TECHNIQUE
NIAID researchers have developed a new method for visualizing in great detail the distribution of cell types in tumors and other complex tissues. The method, called clearing-enhanced 3-D microscopy, or Ce3D, may help researchers evaluate how well immunotherapies target hard-to-treat cancers. Currently available tissue-clearing methods have substantial limits to how many cell types can be characterized and are often very difficult to use, hampering researchers’ ability to obtain a comprehensive understanding of how a patient’s immune system is affecting a tumor. Other current methods permit users to count many different cell types but involve slicing or grinding up the tissue, losing critical information about the 3-D organization of tumor and immune cells in the cancer. By contrast, Ce3D allows researchers to keep the biopsied tissue intact, mark a large number of different cell types with various tracking techniques, and view the results in 3-D. Ce3D is a sophisticated way to obtain a lot of information about a particular tumor, which is critical for researchers trying to evaluate therapies in animal models and physicians evaluating their patients. To see images and videos, click here. (NIH authors: W. Li, R.N. Germain, and M.Y. Gerner, PNAS DOI:10.1073/pnas.1708981114, 2017)
NIMH: ANCIENT ANCESTRAL GENES INFLUENCE MODERN HUMAN BRAINS
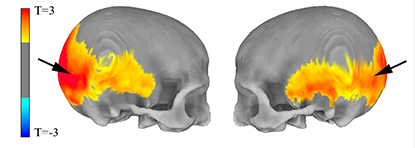
CREDIT: MICHAEL GREGORY, NIMH
NIMH: MRI data show (left) areas of the skull preferentially affected by the amount of Neanderthal-derived DNA and (right) areas of the brain’s visual system in which Neanderthal gene variants influenced cortex folding (red) and gray-matter volume (yellow).
In the distant past, ancestors of modern humans are thought to have mated with their Neanderthal contemporaries, who went extinct 40,000 years ago. A new magnetic-resonance-imaging (MRI) study by NIMH investigators has provided the first direct evidence that this long-ago interbreeding continues to influence our brain structure to this day and potentially our cognitive abilities as well.
Researchers have long studied skulls of Neanderthals to infer their cognitive abilities. Those studies suggest that Neanderthals had more-developed visual systems than modern humans, but at the cost of less-complex brain structures devoted to social interaction. By analyzing the DNA of a large group of participants and scanning their brains with MRI, the NIMH researchers found that subjects whose genes were more similar to those of Neanderthals had skull shapes and brain structures more like members of that ancient human subspecies.
The subjects’ Neanderthal-related genes specifically affected the visual cortex and the intraparietal sulcus in the brain. Because the former is involved in visuospatial abilities and the latter is thought to affect social cognition, the new study suggests that the same tradeoffs the Neanderthal brain made between visual and social capabilities may be made in the brains of modern humans with more Neanderthal-like DNA. Because certain mental disorders, such as the autism-related disorder Williams syndrome, appear to affect both social and visual abilities, the findings could shed light on the genesis of such conditions. (NIH authors: M.D. Gregory, J.S. Kippenhan, D.P. Eisenberg, P.D. Kohn, D. Dickinson, V.S. Mattay, Q. Chen, D.R. Weinberger, Z.S. Saad, and K.F. Berman, Sci Rep 7, 2017; DOI:10.1038/s41598-017-06587-0)
NIA: HOW GLUCOCORTICOIDS TAME INFLAMMATION
Although glucocorticoids are widely used to treat inflammatory conditions, patients often suffer side effects that limit the therapeutic use. Strategies to overcome this problem have been hampered because the cellular actions of glucocorticoid receptor (GR), the molecular target of glucocorticoids, are still debated. NIA researchers used the latest genomic tools and examined the biochemical alterations of the genome in inflammatory cells. The study yielded many surprises, among which was the importance of GR’s ability to turn on genes that counter inflammatory proteins. Conventional wisdom held that GR works by turning down inflammatory genes. This study cautions that the pharmaceutical industry and researchers should re-assess the historical focus on developing synthetic drugs that selectively promote the gene-repressing activity of GR. The gene-inducing activity in GR is crucial for boosting the natural inhibitors of inflammatory factors. According to the researchers, next-generation steroids should keep or enhance this activity of GR for robust anti-inflammatory action while minimizing side effects. (NIH authors: K. Oh, H. Patel, R.A. Gottschalk, W.S. Lee, S. Baek, I.D.C. Fraser, G.L. Hager, and M. Sung, Immunity 47:298–309.e5, 2017, DOI:10.1016/j.immuni.2017.07.012)
NCI: RESEARCHERS IDENTIFY ESSENTIAL GENES FOR CANCER IMMUNOTHERAPY

CREDIT: JONATHAN BAILEY, NHGRI
NCI researchers have identified genes that are essential for cancer cells to be killed by T cells.
A new study, led by NCI and including investigators from several other institutions, identified genes that are necessary in cancer cells for immunotherapy to work. NCI researchers previously showed that the infusion of a large number of T cells can trigger a complete regression of cancer or directly recognize and kill tumor cells. But some tumor cells are resistant to such destruction. In the current study, the researchers used CRISPR-Cas9 gene-editing technology to knock out every known protein-encoding gene in the human genome and then tested the ability of gene-modified melanoma cells to respond to T cells. They found more than 100 genes that may play a role in facilitating tumor destruction by T cells. This gene list could serve as a blueprint to study the emergence of tumor resistance to T-cell-based cancer therapies. (NIH authors: S.J. Patel, R.J. Kishton, A. Eidizadeh, S.K. Vodnala, M. Cam, J.J. Gartner, L. Jia, S.M. Steinberg, T.N. Yamamoto, A.S. Merchant, G.U. Mehta, A. Chichura, E. Tran, R. Eil, M. Sukumar, E.Perez Guijarro, C.Day, P. Robbins, S. Feldman, G. Merlino, and N.P. Restifo, Nature DOI:10.1038/nature23477, 2017)
NIAAA: ALDOSTERONE LINKED TO ALCOHOL-USE DISORDER
A new study, led by NIAAA scientists, demonstrates that aldosterone, a hormone produced in the adrenal glands, may contribute to alcohol-use disorder (AUD). The research was conducted in collaboration with a team of investigators in the United States and Europe. Aldosterone helps regulate electrolyte and fluid balance by binding to mineralocorticoid receptors (MRs), which are located throughout the body. In the brain, MRs are mainly located in the amygdala and the prefrontal cortex—two key brain areas involved in the development and maintenance of AUD. In AUD, amygdala dysfunction heightens activation of brain stress systems, resulting in anxiety and other negative emotions, whereas disruption of the prefrontal cortex impairs executive control systems involved in the ability to make decisions and regulate one’s actions, emotions, and impulses.
The new report describes three separate studies, conducted with nonhuman primates, rats, and humans, that investigated the potential contribution of the aldosterone-MR pathway to AUD. In nonhuman primates, the researchers found that animals that self-administered alcohol every day for six to 12 months had significantly higher blood aldosterone concentrations compared with the concentrations measured before alcohol administration. The researchers also found that in the amygdala, lower MR gene expression–the process of making functional MR protein from the MR gene–was associated with increased alcohol drinking in the animals.
In the rat model of AUD, the researchers found that lower MR gene expression in the amygdala was associated with increased anxiety-like behavior and compulsive drinking compared with rats not exposed to alcohol. Conversely, higher MR gene expression in the amygdala was associated with decreased anxiety and less compulsive alcohol drinking. Overall, these findings suggest that higher MR gene expression in the amygdala may protect against compulsive drinking or, conversely, that lower MR gene expression may increase vulnerability for anxiety-related compulsive drinking in the rat AUD model.
In a human study of about 40 individuals undergoing treatment for AUD, the researchers found that blood aldosterone concentrations were higher in individuals who continued drinking during the 12-week period than in those who were abstinent during the same time frame. The researchers also found that increasing blood aldosterone concentrations correlated with increasing levels of both alcohol craving and anxiety.
Taken together, the researchers conclude that the findings provide support across three different species for a relationship between alcohol misuse, AUD, and specific changes in the aldosterone-MR pathway marked by increased circulating aldosterone and decreased MR gene expression in the amygdala. Future studies should further investigate the mechanisms underlying the relationship between alcohol drinking and the aldosterone-MR pathway and whether this pathway might be targeted for the development of new pharmacotherapies for AUD, according to the researchers. (NIH authors: C.L. Haass-Koffler, R. Lee, L. Leggio, Mol Psychiatry, DOI:10.1038/mp.2017.97)
NIAID: NEW B-CELL PROTECTIVE ROLE IN VIRUS INFECTION
B cells have long been known to generate antibodies in the immune system to fight virus infection. Now NIAID scientists, using a mouse model of retroviral infection, have identified another B-cell role: They protect the host by limiting the response of T cells, a different immune-system fighter that can be overly aggressive and damaging. (NIH authors: T.C. Moore, L.M. Gonzaga, J.M. Mather, R.J. Messer, and K.J. Hasenkrug, mBio 8:e01122-17; DOI:10.1128/mBio.01122-17)
NIEHS: FEMALE MOUSE EMBRYOS REMOVE MALE REPRODUCTIVE SYSTEMS
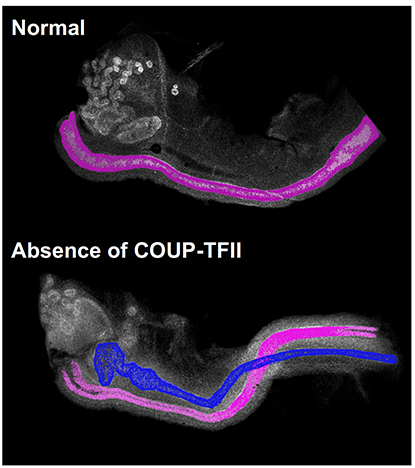
CREDIT: NIEHS
The normal female mouse embryo (top) contains only the female reproductive tract, highlighted in pink. The female mouse embryo without COUP-TFII (bottom) has both male, in blue, and female reproductive tracts.
A protein with the unwieldy name of chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) determines whether a mouse embryo develops a male reproductive tract, according to NIEHS researchers and their colleagues at Baylor College of Medicine (Houston). The discovery changes the long-standing belief that an embryo will automatically become female unless androgens, or male hormones, in the embryo make it male. Since the 1950s, scientists have believed that androgens produced by embryonic testes promote the survival of the male reproductive tract. The scientific consensus favored a female-by-default scenario in which the absence of androgens in female embryos resulted in the breakdown of the male reproductive tract. However, the current work demonstrated that female embryos actively promote the elimination of the male tract through the action of COUP-TFII. Without COUP-TFII, the mice are born intersex, or having both male and female reproductive tracts. The researchers will continue to study how the embryo develops a functional reproductive system. They plan to use mouse models to examine how birth defects of the reproductive system originate. These birth defects lead to disorders of sexual development, which include common defects such as cryptorchidism, or undescended testicles, as well as the genetic disorders Klinefelter syndrome and Turner syndrome. (NIH authors: F. Zhao, H.L. Franco, K.F. Rodriguez, P.R. Brown, and H.H.C. Yao, Science 6352:717–720, 2017; DOI:10.1126/science.aai9136)
NHGRI AND OTHERS: GENE UNDERLYING RARE MUSCLE DISORDER
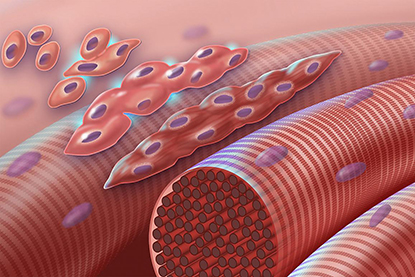
CREDIT: NHGRI
The graphic depicts normal myoblasts fusing together to form muscle cells with more than one nucleus. The cascade is disrupted in Carey-Fineman-Ziter syndrome because of a defect in the membrane protein Myomaker (encoded by the MYMK gene), which is required for cell-cell fusion.
Researchers from across the NIH and other American and international institutions teamed up to identify the genetic mutations that cause Carey-Fineman-Ziter syndrome (CFZS), an extremely rare congenital muscle disease. Sequencing the protein-coding genes in three unrelated families with members who have the disorder revealed that all three families have recessive mutations in the MYMK gene, which governs an important step in muscle development. The scientists also used the CRISPR-Cas9 gene-editing system to create zebrafish (Danio rerio) with MYMK mutations. The fish had muscle abnormalities that were similar to those in human patients. In the fish, the abnormalities were corrected by injecting them with the protein produced by a normal human MYMK gene, suggesting a possible method for treating the condition. Clinicians can also now screen for those MYMK mutations in patients with inherited muscle disorders, leading to faster and more accurate diagnoses. The study provides further insight into the process of muscle development and the array of symptoms experienced by CFZS patients. (NIH authors: C. Van Ryzin, C.R. Ferreira, A. Swift, P.S. Chines, F.S. Collins, L.L. Bonnycastle, N. Narisu, I. Manoli, et al., Nature Communications 8:Article number 16077, 2017; DOI:10.1038/ncomms16077)
NIAID: DISCOVERY OF POTENTIAL BROAD-SPECTRUM ANTIVIRAL
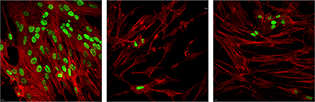
CREDIT: NIAID
The spread of herpes simplex virus infection (green, top) is suppressed in cells treated with EZH2/1 inhibitors.
After herpesviruses infect a cell, their genomes are assembled into specialized protein structures called nucleosomes. Many cellular enzyme complexes can modulate these structures to either promote or inhibit the progression of infection. NIAID scientists studying how one of these complexes (enhancer of zeste 1 and zeste 2 polycomb repressive complex 2 subunit, or EZH2/1) regulated herpes simplex virus (HSV) infection unexpectedly found that inhibiting EZH2/1 suppressed viral infection. The research group then demonstrated that EZH2/1 inhibitors also enhanced the cellular antiviral response in cultured cells and in mice. The inhibitors also suppressed human cytomegalovirus, adenovirus, and Zika virus infections in cell culture using human primary fibroblast cell lines. The authors suggest that EZH2/1 inhibitors have considerable potential as broad-spectrum antivirals. (NIH AUTHORS: J.H. Arbuckle, P.J. Gardina, D.N. Gordon, H.D. Hickman, J.W. Yewdell, T.C. Pierson, T.G. Myers, and T.M. Kristie, mBio 8:e01141-17 DOI:10.1128/mBio.01141-17, 2017)
NCI-DCEG, CC: FEASIBILITY OF CANCER-SCREENING PROTOCOL FOR RARE DISORDER
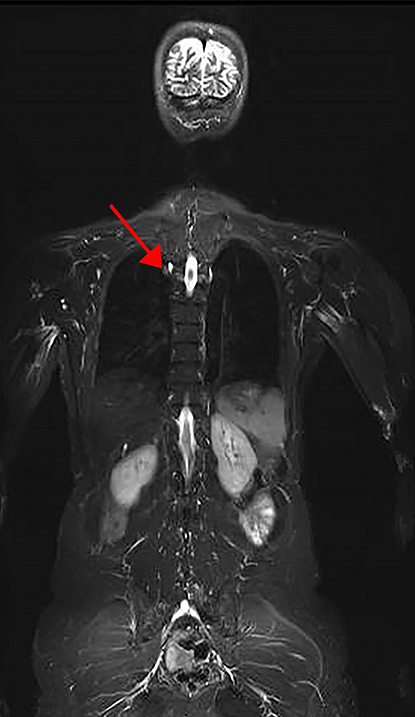
CREDIT: NCI
Part of a representative image of a whole-body MRI of an LFS patient. Arrow denotes lesion found to be lung adenocarcinoma.
In a new study from NCI, researchers found a higher than expected prevalence of cancer at baseline screening in individuals with Li-Fraumeni syndrome (LFS), a rare inherited disorder that leads to a higher risk of developing certain cancers. The research demonstrates the feasibility of a new, comprehensive cancer-screening protocol for this high-risk population. NCI scientists Frederick Pei Li and Joseph F. Fraumeni Jr. first described LFS in 1969. (NIH authors: P.L. Mai, P.P. Khincha, J.T. Loud, R.M. DeCastro, R.C. Bremer, J.A. Peters, C.Y. Liu, D.A. Bluemke, A.A. Malayeri, and S.A. Savage, JAMA Oncol, DOI:10.1001/jamaoncol.2017.1350)
NHGRI: SOCIAL INTERACTION AFFECTS CANCER PATIENTS’ RESPONSE TO TREATMENT
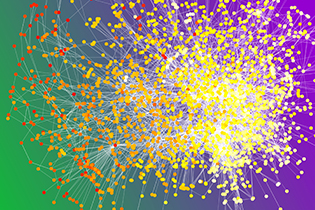
CREDIT: JEFF LEINERT, NHGRI
This image depicts a chemotherapy ward co-presence network. Circles are patients with color based on when they began chemotherapy (white corresponds to January 2000; red corresponds to December 2008). Patients are connected when they were both in the chemotherapy ward at the same time more often than expected by chance.
How well cancer patients fared after chemotherapy was affected by their social interaction with other patients during treatment, according to a new study by researchers at NHGRI and the University of Oxford (Oxford, England). Cancer patients were a little more likely to survive for five years or more after chemotherapy if they interacted during chemotherapy with other patients who also survived for five years or more. Patients were a little more likely to die in less than five years after chemotherapy when they interacted during chemotherapy with those who died in less than five years.
The researchers based their findings on electronic medical records data from 2000 to 2009 from two major hospitals in the United Kingdom’s National Health Service. When patients were around those during chemotherapy who died in less than five years after chemotherapy, they had a 72 percent chance of dying within five years after their own chemotherapy. The best outcome was when patients interacted with someone who survived for five years or longer: They had a 68 percent chance of dying within five years. The researchers’ model also predicted that if patients were isolated from other patients, they would have a 69.5 percent chance of dying within five years.
The researchers didn’t study why the difference occurred, but they hypothesize that it may be related to the stress response. When a person is stressed, stress hormones such as adrenaline are released, resulting in a fight-or-flight response. But if a patient is unable to fight or fly, such as during chemotherapy, these hormones can build up. The researchers also didn’t investigate the impact of visitors on cancer patients undergoing therapy but expect the effect to be similar.
“Positive social support during the exact moments of greatest stress is crucial,” said NHGRI lead author Jeff Lienert, who is also a NIH-Oxford Cambridge Scholars Program fellow. “If you have a friend with cancer, keeping him or her company during chemotherapy probably will help reduce their stress. The impact is likely to be as effective, and possibly more effective, than cancer patients interacting with other cancer patients.” (NIH authors: J. Lienert, C.S. Marcum, and L. Koehly, Netw Sci 1–20, 2017; DOI:10.1017/nws.2017.16)
NICHD, NEI: HIV HIJACKS SURFACE MOLECULE TO INVADE CELL
Researchers at NICHD and NEI have discovered a key step in the process that the human immunodeficiency virus (HIV) uses to inject its genetic material into cells. Working with cultures of cells and tissues, the researchers prevented the invasion process by chemically blocking this step, preventing HIV genetic material from entering cells. The findings could lead to the eventual development of new drugs to prevent HIV infection. To infect a cell, a protein on the surface of HIV binds to molecules on the cell’s surface. This binding process initiates a sequence of events that ends with HIV’s outer membrane fusing with the cell’s membrane. The virus’s genetic material then passes into the cell. The researchers discovered that the binding process activates a protein, called transmembrane protein 16F, that transfers another molecule inside the cell membrane, phosphatidylserine, to the membrane’s outer surface. They believe molecules in the viral membrane bind with the exposed phosphatidylserine on the cell surface to enhance the virus’s fusion to the cell.
The researchers found that blocking the transfer of phosphatidylserine to the cell surface—or attaching another molecule to phosphatidylserine so it can’t bind with HIV—prevents the virus from infecting the cell. Theoretically, the development of drugs that could block each of these steps could provide the basis for treatments to prevent HIV from infecting cells, but much more research is needed. (NIH authors: E. Zaitseva, E. Zaitseva, K. Melikov, A. Arakelyan, M. Marin, R.L. Villasmil, L.B. Margolis, G.B. Melikyan, and L.V. Chernomordik, Cell Host Microbe 22:99–110.e7, 2017; DOI:http://dx.doi.org/10.1016/j.chom.2017.06.012)
NEI, NIAID: EYE MICROBIOME TRAINS IMMUNE CELLS TO FEND OFF PATHOGENS IN MICE
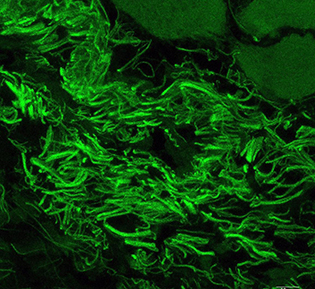
CREDIT: NEI
Corynebacterium mastitidis is a commensal bacterium living on the surface of the eye.
Bugs in your eyes may be a good thing. Resident microbes living on the eye are essential for immune responses that protect the eye from infection, new research shows. The study, led by NEI researchers, demonstrates the existence of a resident ocular microbiome that trains the developing immune system to fend off pathogens. This study is the first evidence that a bacterium lives on the ocular surface long-term and addresses a longstanding question about whether there is a resident ocular microbiome. (NIH authors: A.J. St. Leger, J.V. Desai, R.A. Drummond, F. Almaghrabi, P. Silver, K. Raychaudhuri, M.S. Lionakis, and R.R. Caspi, Immunity 47:148–158.e5, 2017; DOI:10.1016/j.immuni.2017.06.014)
NIAID: ZIKA RESEARCH
NIAID: EXPERIMENTAL ZIKA VIRUS VACCINES RESTRICT IN UTERO VIRUS TRANSMISSION IN MICE
Two experimental vaccines can restrict Zika virus transmission from pregnant mice to their fetuses and can prevent Zika virus–induced placental damage and fetal demise, according to new findings published by NIAID researchers and other partners. The current study is the first animal model study to demonstrate the protection conferred by experimental Zika virus vaccines when administered to female mice before they become pregnant and infected with Zika virus. Further evaluation of the experimental vaccines is warranted, the authors say, as a vaccine that prevents congenital Zika syndrome in people is a critical public-health need. (NIH authors: B.M. Foreman and T.C. Pierson, Cell 170:273–283.e12, 2017; DOI:10.1016/j.cell.2017.06.040)
NIAID: NIH SCIENTISTS TRACK ZIKA VIRUS TRANSMISSION IN MICE
Scientists at NIAID’S Rocky Mountain Labs in Hamilton, Montana, have developed a mouse model to study Zika virus transmitted sexually from males to females, as well as from a pregnant female to her fetus. They are using the model to study how and when the virus is spread, including how the virus crosses the placenta, as well as to investigate potential treatments to block virus transmission. The researchers suppressed interferon (a powerful antiviral protein that inhibits the virus) in specialized laboratory mice that also lack the ability to produce T cells or B cells. These anti-interferon Rag (AIR) mice, have prolonged virus infection in the testes, akin to Zika-infected men. In addition to sexual transmission, the researchers also showed that Zika virus was transmitted vertically from pregnant AIR mice to their fetuses. The researchers found that only some fetuses were infected, suggesting that the placenta may be the most important barrier in preventing Zika virus from reaching the fetus. The group also found that the virus could be detected in fetal tissues other than mouse brain tissue, such as the lymph nodes. Studying how Zika virus is spread in mouse fetuses may help us understand how Zika infection leads to various birth defects in people. (NIH Authors: C.W. Winkler, T.A. Woods, R. Rosenke, D.P. Scott, Sonja M. Best, and Karin E. Peterson, Sci Rep 7:7176; DOI:10.1038/s41598-017-07099-7)
NIEMANN-PICK DISEASE RESEARCH
NCATS, NICHD: HOW AN INVESTIGATIONAL DRUG WORKS IN NIEMANN-PICK DISEASE
CREDIT: NCATS
The compound methyl-beta-cyclodextrin turns on an enzyme, AMPK, triggering a response against a rare genetic disease, NPC1.
An NCATS-led study has demonstrated how an investigational drug works against a rare, fatal genetic disease, Niemann-Pick type C1 (NPC1). They found that a closely related compound will activate the enzyme AMP-activated protein kinase (AMPK), triggering a cellular “recycling” system that helps reduce elevated cholesterol and other accumulated fats in the brains and livers of NPC1 patients. NPC1 occurs when a faulty gene fails to remove cholesterol and other lipids from cells. The lipids accumulate in the spleen, liver, and brain, impairing movement and leading to slurred speech, seizures, and dementia. Patients with NPC1 typically die in their teens, though a late-onset form of the disease affects young adults. The work could lead to a new generation of potential therapies for NPC1 and other similar disorders as well as for neurodegenerative diseases such as Parkinson and Alzheimer diseases. (NIH authors: S. Dai, A.E. Dulcey, X.Hu, C.A. Wassif, F.D. Porter, C.P. Austin, J. Marugan, and W. Zheng, Autophagy 0:1–17; http://dx.doi.org/10.1080/15548627.2017.1329081)
NICHD, NCATS, NIDCD, NINDS, NIMH, CC, NHGRI: EXPERIMENTAL TREATMENT FOR NIEMANN-PICK DISEASE APPEARS SAFE, EFFECTIVE
An NIH-led clinical trial suggests that the drug 2-hydroxypropyl-beta-cyclodextrin (VTS-270) slows the progression of the rare neurological disease Niemann-Pick disease type C1 (NPC1). VTS-270 is being tested under a cooperative research and development agreement between NIH and a pharmaceutical company. The study was a phase 1/2a clinical trial designed to test the drug’s safety and effectiveness. A group of 14 participants, ranging in age from 4 to 23 years, received the experimental drug once a month at NIH for 12 to 18 months. Another group of three participants received the drug every two weeks for 18 months at Rush University Medical Center in Chicago. Initially, participants were divided into groups receiving different doses of the drug, but after observing that the drug was safe and well-tolerated by those receiving the highest doses, the researchers increased dosing for all participants. The progress of the participants was compared with that of a previous group of 21 people with NPC1 enrolled in an earlier study that documented disease progression.
Because NPC1 symptoms result from cholesterol buildup in brain cells, the researchers also measured cholesterol metabolism in the participants’ central nervous system. The results suggest that VTS-270 can improve cholesterol metabolism in neurons, thereby targeting the root of the problem. (NIH authors: E.A. Ottinger, C.P. Austin, X. Xu, J.C McKew, N.Y Farhat, S. Bianconi, L.A Keener, K.A. King, C.C Brewer, A, Soldatos, A. Thurm, B. Solomon, W.J. Pavan, and F.D. Porter, Lancet https://doi.org/10.1016/S0140-6736(17)31465-4)
This page was last updated on Friday, April 8, 2022
