Research Briefs
NIAID: MALARIA SEVERITY NOT DETERMINED SOLELY BY PARASITE LEVELS IN BLOOD
Although malaria kills some 600,000 African children each year, most cases of the mosquito-borne parasitic disease in children are mild. Repeated infection does generate some immunity, and episodes of severe malaria are unusual once a child reaches age 5. However, the relative contributions of such factors as the concentration of malaria-causing parasites in a person’s blood—parasite density—to disease severity and to development of protective immunity are not well understood.

THOMAS WELLEMS, NIAID
The female Anopheles gambiae mosquito can transmit malaria parasites as she takes a blood meal. But the level of parasites in the blood does not determine the severity of malaria, according to a recent NIH study.
To clarify these issues, researchers from the United States and Tanzania regularly examined 882 Tanzanian children beginning at birth and continuing for an average of two years. No simple relationship between parasite density and malaria severity emerged. For example, 253 children had a total of 444 infections characterized by high parasite density and mild symptoms. Of the 102 children who did develop severe malaria at least once while enrolled in the study, almost two-thirds (67) had high parasite density but only mild disease either before or after the episode of severe malaria. Moreover, data from this study suggest that one or two mild episodes of malaria are not sufficient to eliminate the risk of severe malaria, a finding contrary to predictions made by some mathematical models. The researchers note that this prospective study is the first to provide direct evidence that severe malaria risk is stable over several infections. The findings suggest a new approach to malaria vaccine development based on naturally acquired immunity. Such a vaccine would prevent severe disease and death in children, without necessarily reducing exposure to the malaria parasite. (NIAID authors: B.P. Gonçalves, C.-Y. Huang, D.R. Prevots, M. Fried, and P.E. Duffy, N Engl J Med 370:1799–1808, 2014)
NIDA: THE DRUG ECSTASY CAN BE FATAL IN WARM ENVIRONMENTS
A moderate dose of 3,4-methylenedioxymethamphetamine (MDMA; http://www.drugabuse.gov/drugs-abuse/mdma-ecstasymolly), commonly known as Ecstasy or Molly, which is typically nonfatal in cool, quiet environments, can be lethal in rats exposed to conditions that mimic the hot, crowded social settings where the drug is often used by people, a study finds. NIDA scientists have identified the therapeutically relevant cooling mechanism to enable effective interventions when faced with MDMA-induced hyperthermia.
Although MDMA can have a range of adverse health effects, previous studies have shown that high doses of MDMA increase body temperature, whereas results with moderate doses were inconsistent. These results have led some people to assume that the drug is harmless if taken in moderation. However, this study shows that in rats even moderate doses of MDMA in certain environments can be dangerous because it interferes with the body’s ability to regulate temperature.
It is impossible to predict who will have an adverse reaction even to a low dose of MDMA. However, in this study scientists gave the rats low to moderate doses that were shown in past studies to not be fatal. They monitored the rats to determine drug-induced changes in brain and body temperature and in the body’s ability to cool itself through blood-vessel dilation.
When rats were alone and in a room-temperature environment, a moderate dose of MDMA modestly increased brain and body temperature and moderately diminished the rats’ ability to eliminate excessive heat. However, when researchers injected the same dose into rats that were either in a warmer environment or in the presence of another rat in the cage, brain temperature increased, causing death in some rats. These fatal temperature increases were because the drug interfered with the body’s ability to eliminate heat.
These findings further suggest that medical interventions aimed at increasing the efficiency of whole-body cooling by targeting blood-vessel constriction in the skin could be therapeutically relevant for counteracting the development of MDMA-induced hyperthermia. (NIDA authors: E.A. Kiyatkin, A.H. Kim, K.T. Wakabayashi, M.H. Baumann, and Y. Shaham, J of Neurosci 34:7754–7762, 2014)
NIDCR, NIAID: TGF-BETA REGULATES IMMUNE-SYSTEM BALANCE
Without regulatory T cells (Treg cells), you’d likely die from out-of-control inflammation. Treg cells prevent your immune system from attacking your own tissues. NIH scientists reported that they’ve figured out that the indispensable ingredient for making Treg cells in the thymus is transforming growth factor–beta (TGF-beta). One immunological mystery that needed to be solved was how Treg cells in the thymus are generated from progenitor T cells derived in the bone marrow. Another question was why newborns don’t have thymic Treg cells until the third day after birth.
To solve these and other mysteries, many groups are investigating how the Treg cell develops. Through a series of experiments with lab mice, the researchers found that the thymus doesn’t have very much TGF-beta until the third day after birth, and as soon as the TGF-beta concentration is high enough, the Treg-cell factory gears up.
Many in the field have overlooked the normal process of cell self-destruction (apoptosis) in the model of how Treg cells are generated in the thymus. The researchers demonstrated that apoptotic cells lead to TGF-beta production, which in turn leads to Treg-cell development in the thymus. Without TGF-beta, Treg cells are not generated in the thymus.
Because of the new findings at NIH, immunology has a better model to explain Treg-cell development. Such descriptions of the pathways and molecular players involved in Treg-cell development and function might someday identify molecular targets for developing therapies for people with autoimmune diseases, such as Sjögren syndrome and multiple sclerosis, and for people with oral cancer or other types of cancer. (NIDCR authors: J.E. Konkel, W. Jin, B. Abbatiello, W. Chen; NIAID author: J.R. Grainger, Proc Natl Acad Sci U S A 111:E465–73, 2014) [SUBMITTED BY: GERIANN PIAZZA, NIDCR]
NIDDK: STRUCTURE OF BLOOD-CLOTTING RECEPTOR IDENTIFIED
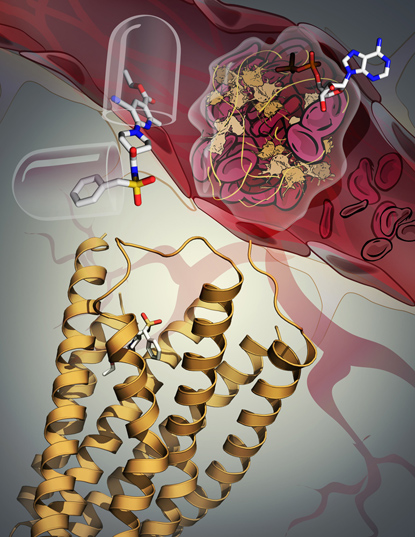
KATYA KADYSHEVSKAYA, SCRIPPS RESEARCH INSTITUTE
The P2Y12 receptor (yellow ribbon) plays a key role in blood-clot formation: When it is stimulated by an adenosine diphosphate (ADP) molecule, blood platelets cluster and clots form (top right). But no one understood how the receptor recognized ADP. In a recent study, however, NIDDK scientists discovered the receptor’s three-dimensional structure and learned that it undergoes pronounced rearrangement when its function is blocked by an antithrombotic drug (upper left).
A team at NIDDK together with scientists at Scripps Research Institute (La Jolla, Calif.), Chinese Academy of Sciences (Shanghai), and the University of Bonn (Bonn, Germany) published two papers in Nature describing their discovery of the three-dimensional structure of a receptor that plays a key role in blood clotting. When the receptor, named P2Y12, is stimulated by adenosine diphosphate (ADP) molecules, platelets cluster and a thrombus (blood clot) forms. The receptor is one of the most prominent clinical drug targets for the inhibition of platelet aggregation. But, until now, scientists have not understood how the receptor works at the molecular level. The study indicates that the P2Y12 receptor’s structure undergoes a pronounced rearrangement when the ADP is replaced by an antithrombotic drug.
Antithrombotic drugs are used to limit the formation of blood clots that can lead to heart attacks and strokes, two leading causes of death in the United States. Such drugs represent a multibillion-dollar market. Understanding in detail how the P2Y12 receptor responds to drug molecules is the first step toward improving antithrombotic drugs and developing potential treatments for nervous system disorders, chronic pain, and other conditions. (NIDDK authors: Z. Gao, S. Moss, S. Paoletta, E. Kiselev, K. Jacobson, Nature 509:115–118, 2014; NIDDK authors: Z. Gao, S. Paoletta, K. Jacobson, Nature 509:119–122, 2014) [SUBMITTED BY: KRYSTEN CARRERA, NIDDK]
This page was last updated on Wednesday, April 27, 2022
