Extending Organ Survival
A Path Toward Animal-to-Human Transplants
Genetically modified pigs may one day offer new hope to thousands of people waiting for heart transplants, thanks in large part to an NIH intramural research team led by Muhammad Mansoor Mohiuddin at the National Heart, Lung, and Blood Institute (NHLBI).
“At any given time, about 3,000 people are on the waiting list for a heart transplant,” said Mohiuddin, who is chief of the Transplantation Section in NHLBI’s Cardiothoracic Surgery Research Program. “But only 2,000 donor hearts become available each year. Human organs alone will never be able to meet this demand.” Many people on the transplant waiting list will die before they receive a replacement heart, he said.
But one day, clinicians may be able to transplant pig hearts into people either as a substitute for human donor organs or as a “bridge” for patients awaiting a human heart transplant.
The use of animal organs in humans, or xenotransplantation, is a potential alternative to conventional transplantation of human donor organs. Artificial hearts might one day provide another alternative, but for now they pose risks such as blood clots, internal bleeding, infection, and mechanical malfunctions.
Pigs are an ideal animal source for xenotransplantation because their organs are similar in size to those of humans; there are fewer ethical issues because pigs are used for food; their breeding cycle is short; and it’s relatively easy to genetically modify them.
A significant obstacle, however, is that the pig and human immune systems are quite different, and a pig heart would be rejected within minutes. Mohiuddin and his team, however, are overcoming this barrier through a combination of genetic engineering and specialized drugs.
The genetic modifications allowed the researchers to use novel drugs to suppress parts of the immune system. These drugs avoid complications associated with traditional pharmacological approaches to preventing rejection, which suppress the entire immune system and put transplant recipients at greater risk for infection and disease.
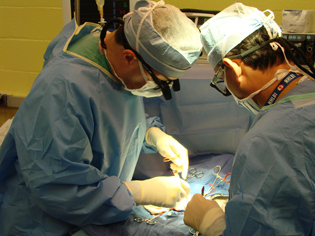
ROBERT F. HOYT, NHLBI
NHLBI surgeon–researchers Muhammad Mohiuddin and Philip Corcoran perform a heart-transplantation procedure. Their work has extended pig-heart graft survival in baboons from mere minutes to 600 days.
The research team collaborated with Revivicor Inc., a Virginia-based regenerative-medicine company, to develop a three-part approach: knocking out certain pig genes for an antigen that stimulates an immune response; inserting a human gene, hCD46, into the pig to prevent activation of the recipient’s innate immune system that would otherwise kill foreign cells including those from a heart graft; and altering the genes responsible for proteins that prevent blood clots in pigs but are incompatible with human blood.
To test the pig hearts, the researchers placed each into a baboon’s abdomen and surgically connected two major blood vessels from the pig heart with two corresponding abdominal vessels in the baboon. Using this method, blood flows through all four chambers of the heart as well as its arteries and veins. The transplanted heart beats but doesn’t replace the baboon’s heart, which continues to function normally. This procedure allowed the researchers to overcome known obstacles from graft rejection while minimizing the risk to the baboons.
The baboon immune system is similar to that of humans and gives researchers the ability to assess whether new transplant techniques are promising enough for human testing. Ethical considerations, federal law, and NIH policy require responsible care and use of research animals. Mohiuddin’s team designed their studies to be sure the baboons survived even if the transplanted cardiac tissue was rejected.
In December 2013, Mohiuddin and his team were the first to demonstrate that interspecies transplanted hearts could survive more than a year in large animals; previously reported survivals of such transplanted hearts were less than four months (Am J Transplan 14:488–489, 2014 at http://onlinelibrary.wiley.com/doi/10.1111/ajt.12562/full).
In April 2014, they further reported survival of 600 days (J Thorac Cardiovasc Surg DOI:10.1016/j.jtcvs.2014.06.002 at http://dx.doi.org/10.1016/j.jtcvs.2014.06.002).
The next step, Mohiuddin said, is to fully replace a baboon’s original heart with a genetically engineered pig heart. If he and his intramural colleagues are successful, they could pave the way for xenotransplantation clinical trials for heart patients.
Others involved in the research: NHLBI: Keith Horvath, Avneesh Singh, Philip Corcoran, and Robert Hoyt; NHLBI Animal Surgery and Resources, Office of Research Services, Division of Veterinary Resources (DVR): Marvin Thomas, Michael Eckhaus, Tannia Clark, Billeta Lewis, and DVR technicians.
This page was last updated on Wednesday, April 27, 2022
