NIH Launches New Translational Science Center
In a move to re-engineer the process of translating scientific discoveries into new drugs, diagnostics, and devices, NIH has established the National Center for Advancing Translational Sciences (NCATS). The action was made possible by Congress’s approval of a fiscal year 2012 spending bill and President Obama’s signing of the bill, which includes the establishment of NCATS with a budget of $575 million.
NCATS will serve as the nation’s hub for catalyzing innovations in translational science. Working closely with partners in the regulatory, academic, nonprofit, and private sectors, NCATS will strive to identify and overcome hurdles that slow the development of effective treatments and cures. Under the current system it can take almost 15 years for a discovery to be translated into an approved new drug. But the failure rate is more than 95 percent, and the cost per successful drug exceeds $1 billion, after adjusting for all the failures.
“Millions of people are looking to science to deliver new and better ways to detect, treat, and prevent disease,” said NIH Director Francis Collins. “Joining some of the best and brightest minds into NCATS is an important step in making the most of current scientific opportunities, examining the therapeutic development pipeline in a new way, and breaking down some of the barriers to translating discoveries into clinical advances for patients.”
The new center gathers existing NIH programs into an integrated scientific enterprise. The NCATS mission is to develop innovative methods and technologies designed to reduce, remove, or bypass bottlenecks in delivering new drugs, diagnostics, and medical devices to patients with a wide range of diseases and conditions.
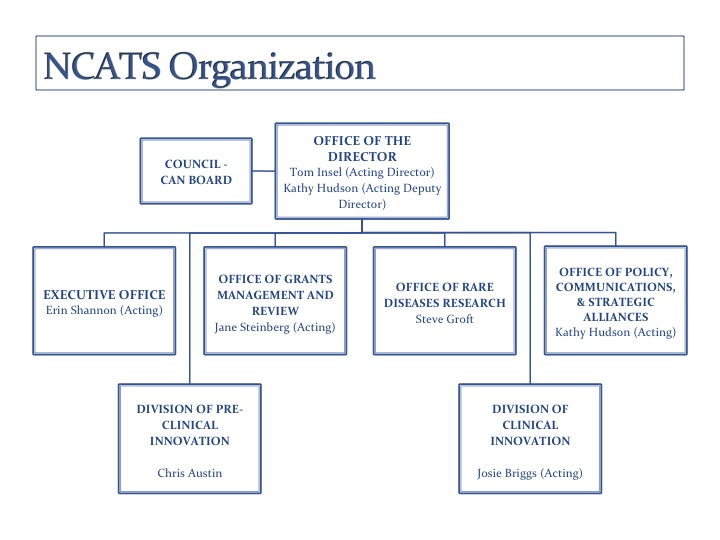
The National Center for Advancing Translational Sciences has no division between intramural and extramural science, but, instead, between pre-clinical research and clinical innovation.
A search is under way for a permanent NCATS director. In the meantime, the new center is being led by NIMH Director Thomas Insel as NCATS acting director and Kathy Hudson, NIH deputy director for science, outreach, and policy, as NCATS acting deputy director.
“Certainly all of us have been the patient or watched loved ones deal with illness, so we know that despite many incredible advances in medicine, there are still limitations,” said Insel. “The goal of NCATS is to fix the parts of the pipeline that aren’t working well to get safer and more effective medicines to patients faster. Who wouldn’t embrace that?”
For more information visit http://ncats.nih.gov.
What Programs Became Part of NCATS?
Much of the planning for NCATS so far has involved coordinating the consolidation of several large, existing programs and offices, some with intramural components. These include the NIH Chemical Genomics Center and the Therapeutics for Rare and Neglected Diseases program, both part of the NIH Center for Translational Therapeutics (NCTT), which is directed by Christopher Austin. NCTT, previously part of NHGRI, is a major intramural component of NCATS. But NCATS is more about collaboration than a dichotomy between extramural and intramural domains. The real division is between preclinical research and clinical innovation; there will be more networking, blurring the line between intramural and extramural.
Programs integrated into NCATS:
- Bridging Interventional Development Gaps, which makes available critical resources needed for the development of new therapeutic agents
- Clinical and Translational Science Awards (previously part of the National Center of Research Resources—NCRR), which funds a national consortium of 60 medical research institutions working together to improve the way clinical and translational research is conducted nationwide
- Cures Acceleration Network, which enables NCATS to fund research in new and ways
- FDA-NIH Regulatory Science, which is an interagency partnership that aims to accelerate the development and use of better tools, standards, and approaches for developing and evaluating diagnostic and therapeutic products
- Office of Rare Diseases Research, which coordinates and supports research on rare diseases
- Components of the Molecular Libraries, which is an initiative that provides researchers with access to the large-scale screening capacity necessary to identify compounds that can be used as chemical probes to validate new therapeutic targets
- Therapeutics for Rare and Neglected Diseases, which is a program to encourage and speed the development of new drugs for rare and neglected diseases
The NCATS budget has been constructed from programs previously located in the NIH Office of the Director, NCRR, and NHGRI. NCRR’s remaining programs will move to other ICs, including NIGMS, NIBIB, NIMHD, and the OD, to take advantage of existing synergies within their portfolios and missions.
This page was last updated on Monday, May 2, 2022
