Why Use Zebrafish to Study Human Diseases?
Scientists use a variety of laboratory techniques to investigate the genetic cause of human diseases. Research often utilizes patients’ cells or tissue samples, but to determine if a mutation in a specific gene can cause a patient’s symptoms, we often need experimental animal models.
While mice and rats have been common choices for modeling human diseases in the past, the use of zebrafish is rapidly gaining popularity. Does this surprise you? Let me explain.
What are zebrafish?
Zebrafish are tropical fresh-water fish in the minnow family. In the wild, they are found in rivers and ponds of India, however they are now often available in pet shops. The name “zebrafish” comes from the horizontal blue stripes on each side of their bodies.

Zebrafish, so named due to their stripes, prefer to live in large groups called shoals.
How can you model a human disease in fish?
Although humans may appear to be extremely different than zebrafish, we are actually much more similar to them than you might think. In fact, 70% of human genes are found in zebrafish.
Moreover, zebrafish have two eyes, a mouth, brain, spinal cord, intestine, pancreas, liver, bile ducts, kidney, esophagus, heart, ear, nose, muscle, blood, bone, cartilage, and teeth. Many of the genes and critical pathways that are required to grow these features are highly conserved between humans and zebrafish. Thus, any type of disease that causes changes in these body parts in humans could theoretically be modeled in zebrafish.
Why use zebrafish when you could use mice?
While mice are evolutionarily more similar to humans because they are mammals, zebrafish have several advantages over their furry competitors.
One important advantage of zebrafish is that the adults are small and prefer to be housed in large groups, or “shoals”. As a result, they require much less space and are cheaper to maintain than mice.

The NIH Zebrafish Core houses hundreds of thousands of zebrafish in a state-of-the-art facility.
Another advantage is that adult zebrafish breed readily (approximately every 10 days) and can produce as many as 50 to 300 eggs at a time. This is quite different from mice as they generally produce litters of one to 10 pups and can only bear approximately three litters in their lifetime. Scientific experiments are generally repeated multiple times in order to prove that the results are accurate, so having an animal that can produce a large number of offspring over and over is helpful.
Zebrafish embryos are also laid and fertilized externally, which allows them to be easily manipulated in a variety of ways. In vitro fertilization can be performed if necessary. The one-cell-stage fertilized eggs can be easily injected with DNA or RNA to permanently modify their genetic makeup in order to generate transgenic or knock-out zebrafish lines. Working with mice in this way is much more complicated. Mouse embryos develop inside the mother, and to access and manipulate them the mother would have to be sacrificed. To keep the embryos alive after fertilizing or injecting them, they would need to be transplanted into another female mouse, as well.
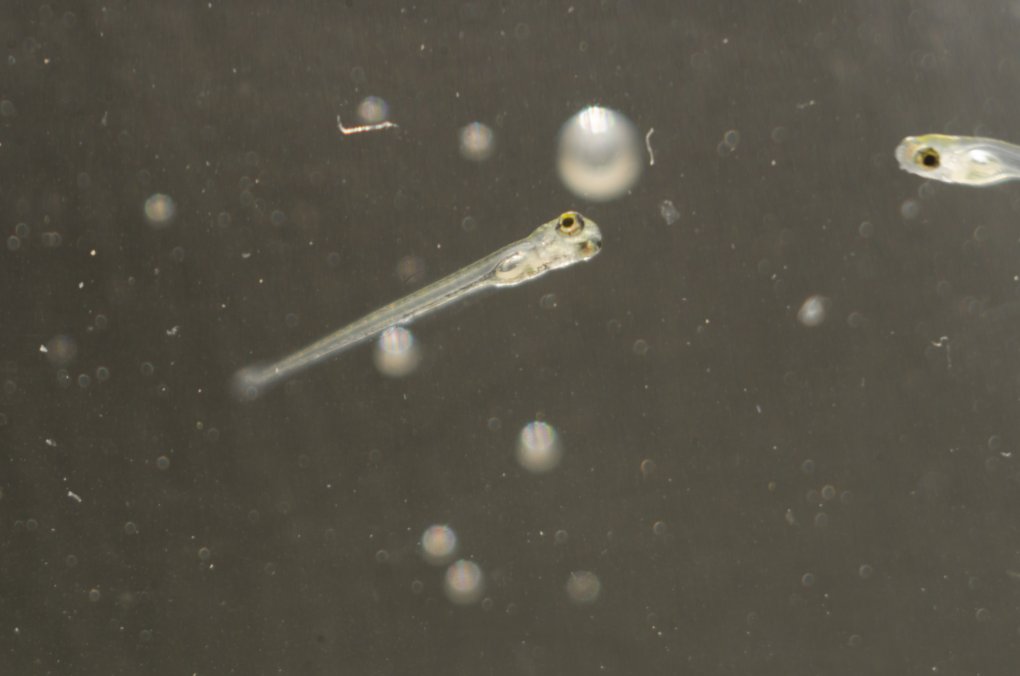
Zebrafish larva, the stage of development from between three and thirty days post-fertilization, grow in length from approximately 3.5 to 8 millimeters.
Furthermore, zebrafish embryos are clear, which allows scientists to watch the fertilized eggs grow into fully formed baby fish under a microscope. Their transparency also enables the visualization of fluorescently labeled tissues in transgenic zebrafish embryos. Mouse embryos are not clear and develop inside the mother, so the observation of live embryo development like that in zebrafish is not possible.
However, there is a limit on what types of diseases can be studied in zebrafish. Human diseases caused by genes that do not exist in zebrafish require a different animal model. Additionally, zebrafish are not useful models for human diseases that mainly take place in a tissue type or body part that zebrafish do not have (e.g., prostate, mammary glands, lungs).
How exactly do you use zebrafish to investigate human diseases?
Often a patient’s DNA is sequenced in order to find a mutation in a gene that could potentially cause his or her disease symptoms. To determine if loss of function of that gene could cause the symptoms seen in the patient, the same gene is mutated or “knocked-out” in zebrafish, and then the fish are examined for similar symptoms. Although it is much more difficult to do, the exact mutation that the patient has can be introduced into zebrafish as well—this is called a “knock-in”.
If one or more of the patient’s symptoms are observed in the zebrafish knock-out or knock-in model, the zebrafish can be used for further studies to help determine why the mutation in that gene causes the disease. For instance, the structure of the muscle fibers can be examined for abnormalities under the microscope if the patient has a muscle disease. Or if the patient’s disease symptoms began during development in utero, knock-out or knock-in zebrafish embryos can be examined for gene expression changes (compared to embryos without the mutation) that could lead to abnormal development. For a patient with a neurological disease, the neurons of knock-out embryos can be fluorescently labeled to see if they form incorrectly.
In addition to utilizing zebrafish disease models to characterize human diseases, researchers can also identify and test new drugs to treat the diseases being modeled. The ability of zebrafish to generate many embryos every time they breed makes them especially useful for high throughput drug screening.
What are some examples of human diseases that have been successfully modeled in zebrafish?
The generation of a knock-out of the dystrophin gene in zebrafish has been shown to closely resemble the severity and progression of the human disease Duchenne muscular dystrophy. Patients with Duchenne muscular dystrophy have been found to carry mutations in dystrophin and demonstrate childhood muscle weakness that gets progressively worse. In both humans and the zebrafish model, the loss of dystrophin gradually leads to necrotic muscle fibers that are replaced by inflammatory cells, fibrosis, and abnormally sized muscle fibers.
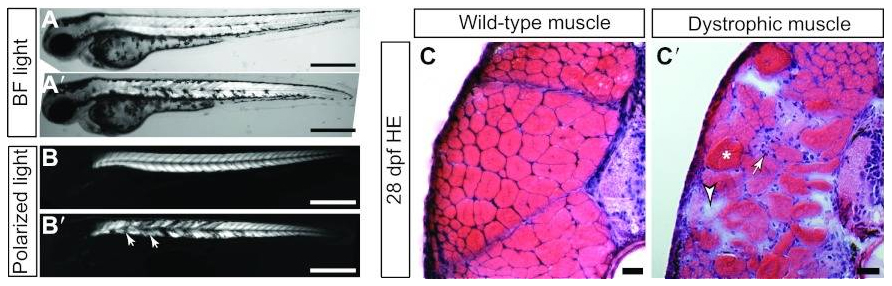
This figure shows visual differences in muscle between wild-type zebrafish larva (A, B, C) and distrophic larva (A’, B’, C’). Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3484855/
Human melanoma has also been successfully modeled in zebrafish. The most commonly identified mutation in human melanomas—a single amino acid change in the gene BRAF—was created in zebrafish to make a knock-in model. Since cancers are caused by a combination of several genetic alterations, this knock-in zebrafish line was used to screen other potential cancer causing mutations. When another commonly observed melanoma mutation of the gene SETDB1 was added to the BRAF knock-in zebrafish, a melanoma rapidly developed. These results helped to establish that SETDB1 is an important gene in melanoma growth.
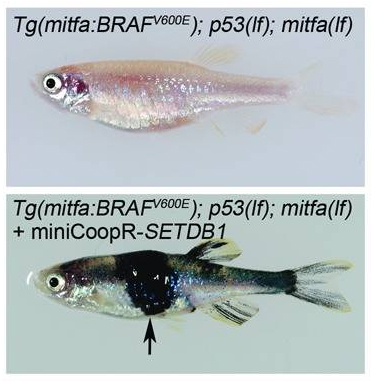
Images of a knock-in zebrafish that expresses the BRAF mutation alone (top) and one that was also injected with a transposon-based vector (miniCoopR) containing a mutant form of the gene SETDB1 (bottom). The addition of the SETB1 mutation resulted in melanoma (indicated by the arrow). Source: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348545/
Those examples of how humans and zebrafish can manifest the same disease despite how different we appear make it is easy to understand why zebrafish are becoming a well-accepted animal model. Here in the NIH Undiagnosed Diseases Program, we perform studies using zebrafish as one of several approaches to investigate the potential involvement of altered genes in our patients’ extremely rare diseases. While mice have been the predominant animal bridge between the bench and bedside in the past, recent studies have demonstrated the potential of zebrafish to serve as a tractable alternative to mice. The timing of the adoption of zebrafish as an emerging model organism could not be better, as mouse studies often fail to translate to humans. Although no animal can perfectly model a human disease, I believe these little striped swimmers have great potential for advancing medical research in the future.
To learn more about how zebrafish contribute to biomedical science and human health, visit the websites for the Trans-NIH Zebrafish Initiative website and the NICHD Zebrafish Core.
Related Blog Posts
This page was last updated on Monday, January 29, 2024
