New Director for the NCATS Stem Cell Translation Laboratory:
Interview with Ilyas Singec

COURTESY: NCATS
Ilyas Singec, who did his postdoctoral training in NINDS more than a decade ago, is the new director of NCATS’s Stem Cell Translation Laboratory.
NIH’s Regenerative Medicine Program (RMP) provides resources and new knowledge to stem-cell researchers to accelerate the development of novel medical applications and cell-based therapies for human disease. To establish and move stem-cell technologies forward via a more centralized effort, NIH has launched a new Common Fund–supported Stem Cell Translation Laboratory (SCTL) within the National Center for Advancing Translational Sciences (NCATS). SCTL will enable researchers across various disciplines and organizations to collaborate and advance the translation of regenerative-medicine applications. Ilyas Singec joined NCATS in 2015 as director of the SCTL.
Induced pluripotent stem cells (iPSCs) are adult somatic cells that have been epigenetically reprogrammed to be in an embryonic stem-cell-like state and are able to differentiate into any cell type. New iPSC-based therapies hold great promise for millions of people suffering from such ailments as Alzheimer disease, diabetes, muscular dystrophy, Parkinson disease, and spinal-cord injury. But there’s a lack of reproducible and well-defined procedures to safely generate, characterize, and differentiate patient-specific iPSCs for preclinical and clinical use.
That’s where Singec and his SCTL staff come in. They are developing new resources and strategies that scientists can use to accelerate the translation of iPSC research into cell therapies and drug discovery.
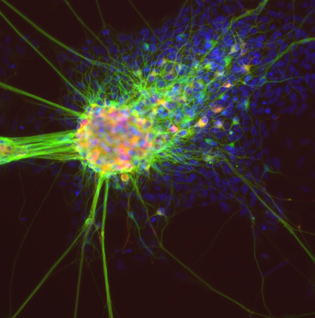
COURTESY: NCATS
Differentiating neurons derived from iPS cells. Green: neuronal marker TUJ1 (beta III tubulin). Red: dopamine neuron marker tyrosine hydroxylase. Blue: nuclear marker DAPI.
The SCTL is using a multidisciplinary collaborative team approach to (1) establish quality-control (QC) standards to define human pluripotency and differentiated cell types; (2) develop methods to assess heterogeneity in cultured cells derived from iPSCs; (3) develop standardized methods to produce mature cells meeting QC standards; and (4) discover, validate, and disseminate small-molecule reagents to replace expensive recombinant proteins, xenogenic material, and undefined media components in cell-differentiation protocols. Current RMP resources (methods and cell lines) are listed on the Common Fund websites at http://commonfund.nih.gov/stemcells/methods and http://commonfund.nih.gov/stemcells/lines.
Singec received his M.D. and Ph.D. degrees respectively from the University of Bonn (Bonn, Germany) and the Albert Ludwig University of Freiburg (Freiburg im Breisgau, Germany) before joining the NIH in 2004 to work as a postdoctoral fellow with Ron McKay in the National Institute of Neurological Disorders and Stroke’s Laboratory of Molecular Biology (2004–2005).
Singec then left NIH and, during the next 10 years, held positions of increasing responsibility in academic research and the pharmaceutical industry. From 2005 to 2008, he was a postdoctoral fellow at the Sanford-Burnham Medical Research Institute in La Jolla, California (recently renamed the Sanford Burnham Prebys Medical Discovery Institute), where he developed the first human iPSC cell lines (2008) and later served as the director of Cell Reprogramming. Subsequently, he was a senior principal scientist, laboratory head, and head of cell technologies at Pfizer in Cambridge, Massachusetts, before returning to NIH in September 2015 as the director of the SCTL.
Following is an edited interview with Singec. For more about him, visit https://ncats.nih.gov/staff/singeci.
What drew you into stem-cell research?
As a physician-scientist, my professional goal is to help patients. At medical school, I first wanted to become a neurosurgeon. But I changed my mind when I started doing laboratory work for my doctoral thesis in neuropathology. I was fascinated by basic questions in neuroscience such as neurotransmission and synaptic plasticity. My first project involved characterizing the molecular and cellular changes that occur in the hippocampus of patients with epilepsy. Around that time, I attended a neuroscience conference in Göttingen, Germany, and was impressed by lectures delivered by German-American biochemist Thomas Südhof, who shared the 2013 Nobel Prize in Physiology or Medicine for research on vesicle trafficking; American neuropsychiatrist Eric Kandel, who shared the 2000 Nobel Prize in Physiology or Medicine for his work on the physiological basis of memory storage in neurons; and American neuroendocrinologist Bruce McEwen.
Eventually, a Nature paper published in 1997 got me interested in adult neurogenesis and neural stem cells (Nature 386:493–495, 1997). Seeing the great potential of stem cells, particularly of pluripotent stem cells, I decided to join NINDS in 2004 for postdoctoral training.
How have your previous positions prepared you to be the SCTL director?
My positions in academia and industry helped me to get a clear understanding of the challenges and opportunities associated with the application of human stem cells for regenerative medicine and drug discovery. Going back to my earliest studies in Germany, I was always very independent and unbiased in my approach to science. At the same time, I always stayed close to laboratory work and raw data and understood the importance of looking at discoveries with my own eyes, ideally through the microscope. I also carefully studied various human and rodent model systems including adult, embryonic, and reprogrammed stem cells. I think having hands-on expertise and transparency is critical to meeting the challenges ahead. In January 2008, I independently generated the first iPSC lines at the Sanford-Burnham. Soon I was able to produce more than 70 iPSC lines from patients with various neurological and psychiatric disorders. In parallel in 2008, I combined three small molecules—dorsomorphin, A83-01, [and] PNU-74654 [collectively, DAP]—to develop a chemically defined and highly efficient six-day neural-induction protocol. These small molecules transiently block BMP [bone morphogenic protein], TGF-beta [transforming growth factor–beta], and WNT signaling. Our “DAP protocol” is now being used by other groups. This idea was inspired by published research (J Cell Sci 117:1269–1280, 2004). Based on this experience, I understand the importance of leading and promoting collaborative team efforts and the commitment to innovation, high standards, and data sharing.
How do you envision the SCTL benefiting stem-cell researchers inside and outside the NIH?
My SCTL team and I will take advantage of the unique resources and environment provided by NIH Common Fund and NCATS in order to help advance the application of human pluripotent stem-cell biology. We envision collaborating with the intramural and extramural research communities on specific projects and key questions so that the iPSC technology can be firmly established for personalized cell therapies, drug discovery, and toxicology testing. Once developed and externally validated, we hope that our assays and protocols will be widely implemented and used. Accordingly, all information and new resources will be shared with the public.
How do NIH researchers go about setting up collaborations with the SCTL?
NCATS has an open-minded scientific culture and track record for setting up successful collaborations. NCATS will operate the SCTL in a similar fashion and leverage collaborations by addressing important questions that align with SCTL goals. We will announce a process for intramural and extramural researchers to apply to become collaborators of the SCTL, including a review process to identify the most promising opportunities. For now, those interested should contact me personally at ilyas.singec@nih.gov. When setting up collaborations, we will discuss timelines, define deliverables, and assign shared responsibilities. This process is important given the laborious and costly trajectory that is typical for human stem-cell work. NCATS is already collaborating with intramural scientists doing stem-cell work. We will build on this experience and also start new collaborative projects with our NIH colleagues.
What equipment and techniques are available now or will be within the next 12 to 18 months?
Over the last couple of years, visionary colleagues at NCATS have established important technological platforms, new assays and small-molecule libraries for drug screening and translational research. The new stem-cell group is fortunate to be able to access these resources, which will play an important role in setting up collaborations. Moreover, NCATS has dedicated more than 4,400 square feet for a new state-of-the-art laboratory space for iPSC research [in Rockville, Maryland]. The newly renovated and outfitted stem-cell laboratory likely will be ready this fall. Apart from high-throughput and high-content screening, major investments will be made in automated cell culture, quantitative biology, and single-cell analysis.
Lastly, how do you like to spend your free time?
I always had an interest in fine arts, classical music, and history. In my limited free time I am trying to explore the rich cultural program that the Washington, D.C., area has to offer. For instance, I recently enjoyed the Gustave Caillebotte exhibition at the National Gallery of Art. I often have Bach’s “Goldberg Variations” performed by Glenn Gould or the Chopin “Nocturnes” playing in the background. A personal goal for next year is to run another half-marathon.
The Regenerative Medicine Program is supported by the NIH Common Fund (https://commonfund.nih.gov/stemcells/index). Common Fund programs are designed to pursue major opportunities and gaps in biomedical research that no single NIH Institute could tackle alone, but that the agency as a whole can address to make the biggest impact possible on the progress of medical research. NCATS focuses on what is common across diseases and the translational process; it emphasizes innovation and deliverables, relying on the power of data and new technologies to develop, demonstrate, and disseminate advancements in translational science that bring about tangible improvements in human health. For more information, visit https://ncats.nih.gov/stemcell or sign up for updates at https://ncats.nih.gov/connect.
This page was last updated on Thursday, April 14, 2022
