Research Briefs
NIEHS: NATURAL PROTEIN POINTS TO NEW INFLAMMATION TREATMENT
COURTESY: NIEHS
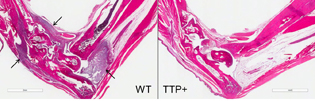
Stained sections of foot joints show that when researchers created two mouse models of rheumatoid arthritis, the wild-type mouse, left, experienced significant inflammation. Arrows point to the presence of inflammatory immune cells in tissues lining the joints. In contrast, the mouse with higher amounts of TTP, right, did not exhibit inflammation.
NIEHS researchers report that increasing the concentration of tristetraprolin (TTP), a naturally produced protein, in mice significantly reduced inflammation or protected the mice from it altogether. The researchers genetically altered ZFP36, the gene that codes for TTP, in mice to produce higher than normal amounts of the protein. The mice were then tested by inducing a disease that has features similar to human rheumatoid arthritis (RA), psoriasis, or multiple sclerosis (MS). Mice with more TTP in their bodies were resistant to the inflammation that accompanied the induction of disease.
The team also found evidence that TTP exerts its beneficial effect by targeting several messenger RNAs (mRNA) that encode cytokines. TTP binds to mRNAs and destabilizes them, resulting in lower concentrations of cytokines and thus decreased inflammation. The results suggest that pharmaceutical compounds, or other therapeutic methods that produce elevated TTP in humans, may offer an effective treatment for some inflammatory diseases, such as RA, psoriasis, and MS. (NIH authors: S. Patial, W.S. Lai, D.J. Stumpo, G.D. Hill, G.P. Flake, and P.J. Blackshear, Proc Natl Acad Sci U S A 113:1865–1870, 2016)
NHGRI, NINDS, NCI, NIA: T-CELL TRANSCRIPTION FACTOR MAY OFFER NEW PATHWAY FOR VACCINE RESEARCH
A team led by NHGRI scientists discovered that the transcription factor T-cell factor–1 (TCF1) may show promise for the development of vaccines. The researchers determined that the presence of TCF1 is necessary for the generation of white blood cells called T follicular helper (TFH) cells in response to a viral infection. These TFH cells then interact with the B cells that actually produce the antibodies. If the TCF1 transcription factor is absent or weakened, the TFH cells—and the antibodies—are either damaged or nonexistent. Although further research is needed, the findings “may help shed light on pathways important for the development of vaccines and immune therapies targeting viral infections,” the authors wrote. (NIH authors: T. Wu, E.A. Moseman, Y. Ji, B. Huang, C. Harly, J.M. Sen, L. Gattinoni, D.B. McGavern, and P.L. Schwartzberg, Cell Rep 12:2099–2110, 2015)
NIMH: CIRCUIT TWEAK BOOSTS SOCIAL MEMORY IN MICE
NIMH researchers have boosted the staying power of a social memory at least 80-fold by stimulating a circuit they discovered in a mouse brain. A male mouse would normally forget a female mouse it had just met within an hour. However, when the circuit was stimulated in a male mouse, it instead remembered the female at least a week later. Researchers genetically primed the circuit to respond to pulses of light in a technique called optogenetics.
The study is the first to enhance social memory by stimulating a specific circuit. The enhancement worked only if the male’s circuit was stimulated while the memory was being formed, not recalled—and only during its first encounter with the female mouse. The memory remained strong even after the male was distracted by the introduction of a second female mouse, which would normally degrade memory of the first female mouse. Based on their previous studies, the team knew that genetically silencing the activity of a receptor for the social behavior–related hormone vasopressin blocks social memory. They also knew that brain expression of the vasopressin 1b receptor is confined mostly to a little-studied part of the hippocampus called CA2 and that blocking CA2 reduces social memory. So the researchers set out to discover the upstream circuitry that triggers release of vasopressin in CA2.
A prime candidate was a set of neurons that project to CA2 from a part of the hypothalamus called the paraventricular nucleus (PVN). The PVN integrates information from external and internal environments to orchestrate stress responses. In the new study, the researchers were able to confirm that vasopressin activity in CA2, triggered by the circuit from PVN, is a key player in social memory, although other vasopressin pathways are also likely involved. The researchers believe that if the same circuitry is at work in the human brain, treatments based on similar targeted brain-pathway stimulation might someday help to improve the relationships of people experiencing social-memory impairment due to dementias and mental illnesses. (NIH authors: A.S. Smith, S.K. Williams Avram, A. Cymerblit-Sabba, J. Song, and W.S. Young, Mol Psychiatry DOI:10.1038/mp.2015.189)
NICHD: POVERTY MAY SLIGHTLY INCREASE CHILDHOOD RISK OF NEUROLOGICAL IMPAIRMENT
Children from low-income environments seem to have a higher risk of neurological impairment than those from more economically secure circumstances, according to a multi-institutional study led by an NICHD researcher. This neurological impairment appears distinct from the risk of cognitive and emotional delays known to accompany early-life poverty. Increased neurological impairment could increase the risk for childhood learning difficulties, attention deficit disorders, and psychological conditions such as anxiety disorders and schizophrenia. The researchers analyzed data from 36,443 participants in the United States Collaborative Perinatal Project, a study of a socioeconomically diverse pregnancy cohort conducted between 1959 and 1974. Children in the study received comprehensive neurological examinations at birth, 4 months, 1 year, and 7 years of age.
Beginning at age 4 months, the chance of having a neurological abnormality was higher in the most disadvantaged children (12.8 percent) compared with the least disadvantaged (9.3 percent). By age 7, the likelihood of a neurological abnormality increased to 20.2 percent among the most disadvantaged compared with 13.5 percent among the least disadvantaged.
Studies indicate that people living in poverty are at higher risk for substance abuse, anxiety, depression, and child abuse, and the authors theorize that these factors could explain the higher rates of neurological impairment their study found for children raised in impoverished environments. Further research into how childhood poverty might contribute to neurological impairment could lead to ways to prevent neurological impairment from occurring. (NIH author: S.E. Gilman, Int J Epidemiol 44:1889–1899, 2015)
NIDCD: DIZZINESS AND BALANCE PROBLEMS COMMON IN U.S. KIDS
More than 1 in 20 children in the United States have a dizziness or balance problem, and only one-third of them had received treatment in the previous year, scientists report. A team led by NIDCD researchers analyzed data on nearly 11,000 children, ages 3 to 17. Parents were asked whether, in the past year, their children had been bothered by symptoms of dizziness or balance problems such as vertigo, unsteadiness upon standing, frequent falls, or other related symptoms. Analyses showed that 5.3 percent of U.S. children (nearly 3.3 million) had dizziness or balance problems. Prevalence increased with age, with 7.5 percent of kids ages 15–17 and 6.0 percent of children ages 12–14 having any dizziness or balance problem. The prevalence was 3.6 percent for children ages 6–8 and 4.1 percent for kids ages 3–5. Nearly 1 in 5 affected kids (18.6 percent, or 600,000 children) had symptoms rated as “moderate,” “big,” or “very big” problems.
Diagnoses made included neurological disorders, ear infections, concussion, malformation of the ear, prescription medications, severe headaches or migraines, and vision problems. Children with hearing difficulties were more likely to have dizziness or balance problems than children with normal hearing. Other risk factors linked to dizziness and balance problems included frequent headaches, certain developmental delays, and occurrence in the previous year of seizures, stuttering or stammering, or anemia. “These findings suggest that dizziness and balance problems are fairly common among children, and parents and providers should be aware of the impact these problems can have on our children,” said NIDCD Director James F. Battey, Jr. “Parents who notice dizziness and balance problems in their children should consult a health-care provider to rule out a serious underlying condition.” (NIH authors: C.M. Li and H.J. Hoffman, J Pediatr PII:S0022-3476(15)01512-7; DOI:10.1016/j.jpeds.2015.12.002.)
NIDA: MARIJUANA-LIKE BRAIN CHEMICAL MAY AFFECT COCAINE ADDICTION
A series of experiments performed by scientists at NIDA and the University of Maryland School of Medicine has revealed how compounds called endogenous cannabinoids produced in the brain influence the rewarding properties of cocaine. Cocaine and other drugs of abuse trigger neurons in the brain’s ventral tegmental area (VTA) to release the “pleasure chemical” dopamine into the nucleus accumbens (NAc), a neural structure involved in reward and addiction. The resulting increase in dopamine concentrations in the NAc leads to the intense cocaine “high.” VTA neurons also inhibit dopamine release from the NAc via a chemical called gamma–aminobutyric acid.
Previous studies have shown that endogenous cannabinoids are involved in this process, though until now their role was unclear. The scientists discovered that cocaine causes the VTA to release an endogenous cannabinoid called 2-arachidonoylglycerol, or 2-AG. They also found that this substance acts via cannabinoid receptor 1 to decrease the amount of GABA released by VTA neurons, thereby increasing dopamine release in the NAc and enhancing the pleasure associated with cocaine use. The results suggest therapeutic interventions that affect the brain’s endogenous cannabinoid system may help cocaine users kick the habit. (NIH authors: H. Wang, T. Treadway, C.R. Lupica, Cell Rep 12:1997–2008, 2015)
This page was last updated on Thursday, April 14, 2022
