Visualizing Infection
New Ways to Image the Immune System
“We live in a dangerous world, constantly bombarded with bacteria, viruses, fungi, or parasites,” said Ronald Germain at the annual G. Burroughs Mider Lecture, held in December. “How does the immune system protect against adverse unpredictable disease entities at unanticipated sites in the body?”
Fighting off infection is an incredibly complex process. There’s not one type of immune cell, but many. They swarm. They dance. They work in sync. Germain, who’s chief of the Laboratory of Systems Biology and of the Lymphocyte Biology Section at the National Institute of Allergy and Infectious Disease (NIAID), uses cutting-edge imaging techniques to investigate the intricate movements and positioning of immune cells.
“In contrast to almost all other tissues in an adult, the immune system is unique [because] there are cells moving all around in many tissues and organs, touching each other, transmitting signals, then going apart and changing their function,” said Germain. “It’s essentially impossible to access these key aspects of immune behavior without doing imaging.”
His lab was one of the first to use a technique called two-photon intravital imaging, which uses laser illumination to see deep into living tissue without causing the damage done by traditional confocal fluorescence microscopy. Germain collaborates with other scientists, such as Pamela Schwartzberg (National Human Genome Research Institute), who want to make use of his imaging expertise. Schwartzberg studies the action of immune cells in her mouse model that has a mutation in a gene that makes humans more susceptible to lymphomas and lymphoproliferative disease.
The T cells and B cells interact effectively in artificial environments. But Germain’s intravital-imaging technique revealed that in living tissue, where immune cells are constantly moving, the mutant immune cells couldn’t interact long enough with one another to adequately coordinate their activity (Nature 455:764–770, 2008).
“We only could see that [problem] by measuring the duration of the interactions [among] the cells,” Germain said. “You only could do that if you could do dynamic, in vivo imaging.”
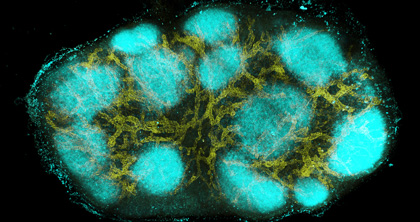
CREDIT: W. Li, NIAID
Image of an intact mouse lymph node created using a new clearing and staining technique called clearing-enhanced 3D (Ce3D) imaging, which was developed by Weizhe Li, Michael Gerner, and Ronald N. Germain, in the Lymphocyte Biology Section, Laboratory of Systems Biology, NIAID. The image shows high endothelial venules and capillaries (in yellow) and B cells in primary follicles (in cyan).
Germain’s group has also pioneered two other imaging technologies—histocytometry and three-dimensional (3D) imaging. Histocytometry, developed by Michael Gerner in Germain’s laboratory, is an analytical microscopy method for visualizing and quantifying complex cell populations directly in tissue. The technique is based on multiplexed antibody staining, tiled high-resolution confocal microscopy, voxel gating, volumetric cell rendering, and quantitative computer analysis, yielding previously unobtainable levels of information about immune cells in complex environments.
In a proof-of-concept experiment, Germain’s lab mapped the locations of different types of dendritic cells in the mouse lymph node (Immunity 37:364–376, 2012). The study showed that histocytometry can identify the type and number of cells in a sample just as effectively as flow cytometry but is superior at analyzing the characteristics of cells that are difficult to remove from their native tissues.
In a more recent paper, Germain’s team examined the role of regulatory T cells in curtailing the immune response and found that these inhibitory cells act in small, localized clusters to prevent T cells activated by self-antigens from damaging the body (Nature 528:225–230, 2015). The finding that auto-reactive cells become partially activated before they are inhibited sheds light on why autoimmune disease follows so quickly when the regulatory T cells cease to function properly.
Germain’s team also developed a type of 3D imaging called clearing-enhanced 3D (Ce3D) imaging, which can be used for both human and animal studies without distorting the shape of the cells, interfering with fluorescent protein activity, or preventing antibody-mediated staining as some other 3D-imaging methods do. Because it takes about half a day to create a 3D image of a single sample using the Ce3D method and current commercial microscopes, Germain is working with Hari Shroff of the National Institute of Biomedical Imaging and Bioengineering to develop instruments that could produce data in less than a tenth of the time.
“I’ve presented these new approaches [histocytometry and Ce3D] at meetings over the last couple of months,” said Germain. “We have to keep ‘beating people off with a stick’ because of the number of groups that want to collaborate.”
To enable many investigators, both basic and clinical, to use these methods, Germain is attempting to establish a new center for tissue imaging at NIH. The center would include research microscopes for developing new imaging methods, as well as several identical high-end instruments needed to quickly produce high quality histocytometry and Ce3D data from large sample numbers for both animal-based and human clinical trial studies. He also plans to hold courses in the facility to train other researchers to use this technology.
“We need money for instruments, we need resources for staff, and we need space to accomplish these goals,” said Germain. “Hopefully the NIAID and larger NIH community will come together to support this center. [It] could make a real difference in understanding things like the immune response to cancer, how vaccine adjuvants work, and how autoimmune diseases develop and damage tissues.”
To watch a videocast of Germain’s talk (“Imaging Immunity”) at the annual WALS G. Burroughs Mider Lecture, held in the Masur auditorium on December 9, 2015, go to http://videocast.nih.gov/launch.asp?19375.
This page was last updated on Thursday, April 14, 2022
